Sulfydryl functionalized organic aromatic carboxylic acid ligand and preparation method and application thereof
A technology of aromatic carboxylic acid and functionalization, which is applied in the fields of mercaptan preparation, thioether preparation, organic chemistry, etc., can solve the problem of insufficient mercapto group content, and achieve the effect of simple preparation method, good performance and less preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
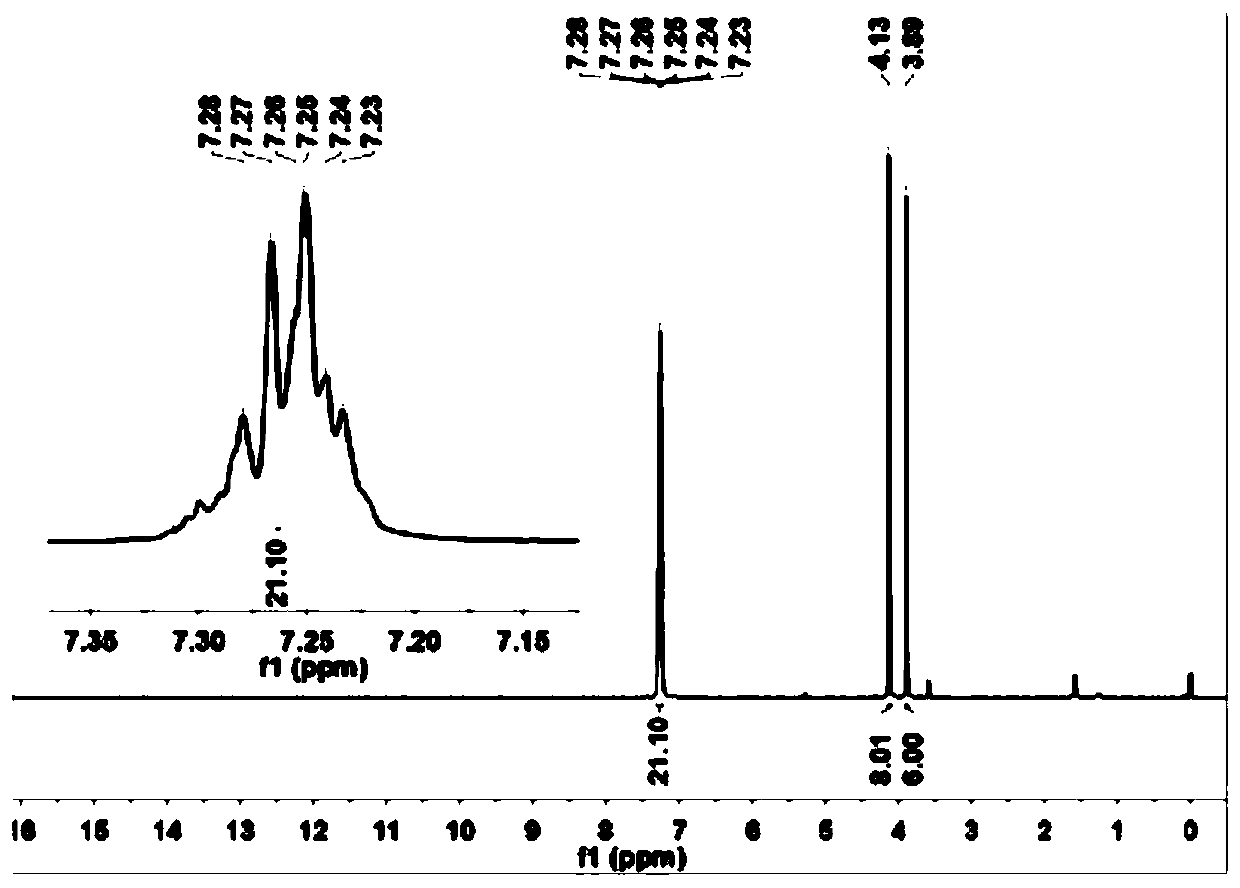
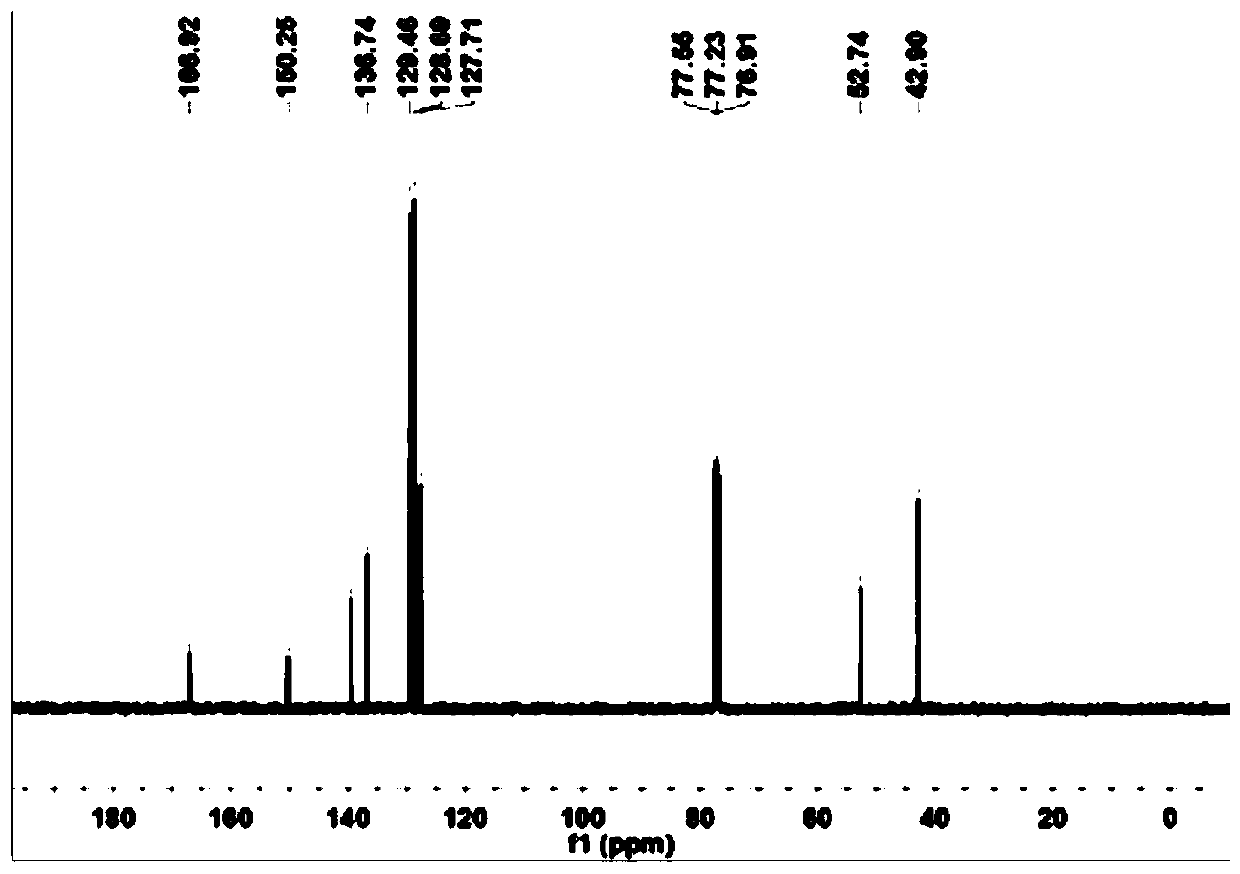
Image
Examples
Embodiment 1
[0047] This example is the preparation of the precursor of sulfur-based functionalized organic aromatic carboxylic acid ligands
[0048] 1) in N 2 Under protection, the raw material methyl tetrafluoroterebenzoate M1 (2.7 g) was weighed and added into a 250 mL two-neck round bottom flask. Weigh benzyl mercaptan (27.2g) and potassium carbonate (50g) into a 250mL two-necked round bottom flask respectively. The round bottom flask was attached to the Schlenk line. Transfer 90 mL of degassed anhydrous N,N-dimethylformamide solution to a flask, and then stir the reaction at 25° C. for 48 h.
[0049] 3) Stirring was stopped, then the reaction mixture was poured into water (150 mL), the aqueous solution was extracted (3 x 80 mL) with ethyl acetate, and the ethyl acetate was combined. The extracted ethyl acetate was washed with water (3×100 mL), followed by anhydrous MgSO 4 The ethyl acetate was dried and then removed with a rotary evaporator. The crude product was obtained as a pa...
Embodiment 2
[0056] This example is the preparation of thiol-functionalized organic aromatic carboxylic acid ligands
[0057] 1) in N 2 Under protection, weigh 300mg of M2 and add it to a 50mL reaction bottle. Connect the reaction eggplant bottle to the gas line conduit, then evacuate it and fill it with N2, repeat three times, then freeze the reaction eggplant bottle with liquid nitrogen, slowly add 600mg of aluminum trichloride under the protection of nitrogen, and then raise the temperature to room temperature (25°C) , Stir the reaction for 12h.
[0058] 2) After stopping the reaction, ice-bath the reaction system, and under the protection of nitrogen, add ice cubes into the reaction eggplant bottle, a large amount of solids precipitate out, and vigorously stir for 30 minutes. Filter under reduced pressure, wash the filter residue with a large amount of water, wash the residual by-products, dry in the air, and vacuum-dry to obtain tetramercaptoterephthalic acid, which is recorded as M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com