Preparation process of dexmedetomidine hydrochloride injection
A technology for preparing dexmedetomidine hydrochloride and a preparation process, which is applied in the field of preparation of liquid medicine for injection, can solve problems such as short validity period, difficulty in controlling the validity period, and changes in the pH value of the liquid medicine, and achieve improved stability, easy product quality, The effect of stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation process for dexmedetomidine hydrochloride injection, comprising the following steps:
[0023] A. Soak 1ml of neutral borosilicate ampoule in 0.1M hydrochloric acid solution and ultrasonically clean it for 1 hour, then dry it with compressed air, rinse it with purified water and water for injection in sequence, and then sterilize and dry it in a tunnel oven at 330°C to obtain acid Dispose of neutral borosilicate ampoules, spare;
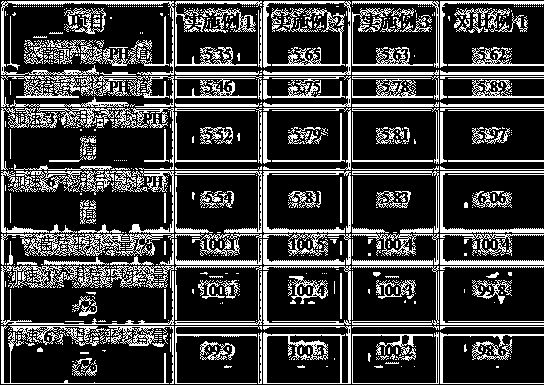
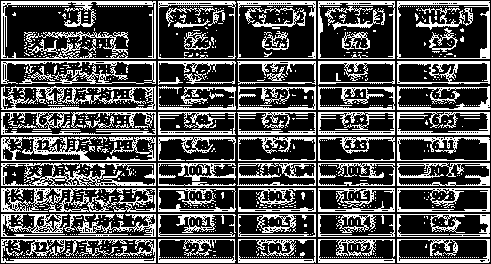
[0024] B. Weigh 59g of dexmedetomidine hydrochloride and 4.5kg of sodium chloride, add 500L of water for injection to dissolve, and stir well to obtain pH 5.2, containing 0.1mg / ml of dexmedetomidine hydrochloride and 9mg / ml of sodium chloride. The crude product of dexmedetomidine hydrochloride injection of ml;
[0025] C. The crude product of dexmedetomidine hydrochloride injection is filtered through 0.45μm and 0.22μm filter membranes successively, and filled in 1ml acid-treated neutral borosilicate ampoules with a filling volum...
Embodiment 2
[0028] A preparation process for dexmedetomidine hydrochloride injection, comprising the following steps:
[0029] A. Soak 2ml of neutral borosilicate ampoule in 0.1M hydrochloric acid solution and ultrasonically clean it for 1 hour, then dry it with compressed air, rinse it with purified water and water for injection in sequence, and then sterilize and dry it in a tunnel oven at 330°C to obtain acid Dispose of neutral borosilicate ampoules, spare;
[0030] B. Weigh 59g of dexmedetomidine hydrochloride and 4.5kg of sodium chloride, add 500L of water for injection to dissolve, stir well to obtain pH 5.5, containing 0.1mg / ml of dexmedetomidine hydrochloride, and 9mg / ml of sodium chloride. The crude product of dexmedetomidine hydrochloride injection of ml;
[0031] C. The crude product of dexmedetomidine hydrochloride injection is filtered through 0.45μm and 0.22μm filter membranes successively, and filled into 2ml acid-treated neutral borosilicate ampoules with a filling volume...
Embodiment 3
[0034] A preparation process for dexmedetomidine hydrochloride injection, comprising the following steps:
[0035] A. Soak 2ml of neutral borosilicate ampoule in 0.1M hydrochloric acid solution and ultrasonically clean it for 2 hours, then dry it with compressed air, rinse it with purified water and water for injection in sequence, and then sterilize and dry it in a tunnel oven at 330°C to obtain acid Dispose of neutral borosilicate ampoules, spare;
[0036] B. Weigh 118g of dexmedetomidine hydrochloride and 9kg of sodium chloride, add 1000L of water for injection to dissolve, stir well to obtain pH5.5, containing 0.1mg / ml of dexmedetomidine hydrochloride, and 9mg / ml of sodium chloride crude dexmedetomidine hydrochloride injection;
[0037] C. The crude product of dexmedetomidine hydrochloride injection is filtered through 0.45μm and 0.22μm filter membranes successively, and filled into 2ml acid-treated neutral borosilicate ampoules with a filling volume of 2ml±0.1ml, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com