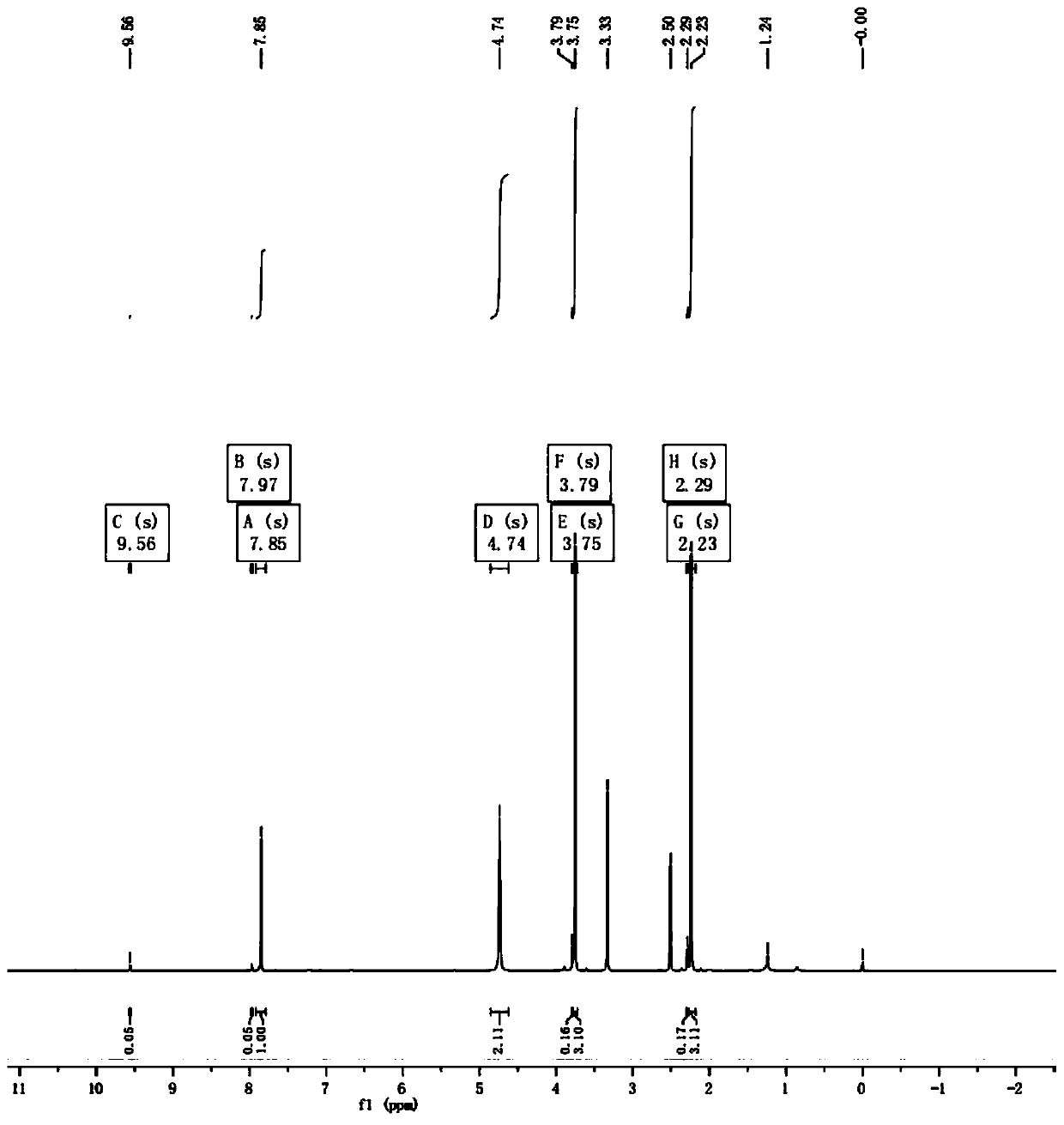
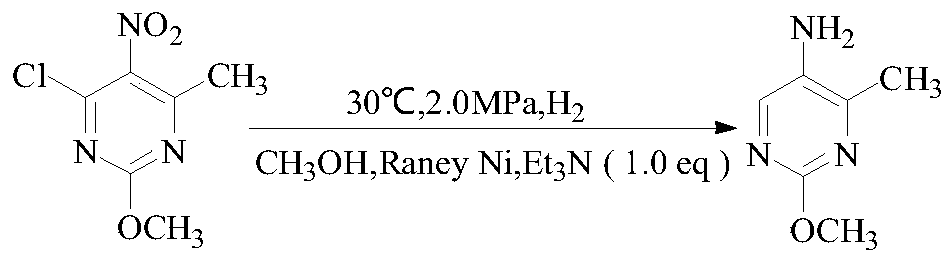Preparation method of 2-methoxy-5-amino-4-methylpyrimidine
A technology of methyl pyrimidine and methoxy group, applied in the field of fine chemical intermediates, achieves the effect of simple synthesis process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
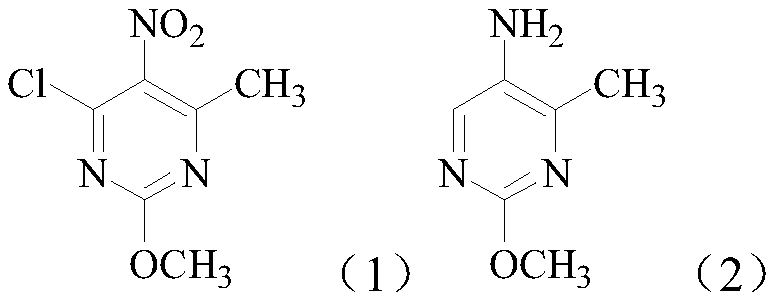
[0016] The present embodiment is the preparation method of 2-methoxy-5-amino-4-methylpyrimidine, according to the following reaction formula:
[0017]
[0018] The specific experimental process is as follows:
[0019] Add 2-methoxyl-5-nitro-4-methyl-6-chloropyrimidine 8g (0.04mol, 1.0eq) into the autoclave, add methanol 80ml, nickel 0.4g, triethylamine 4g (0.04mol, 1.0eq), at 30°C and 2.0MPa, hydrogen gas was introduced, stirred for 2h, the reaction was completed, filtered, the filtrate was concentrated, 20ml of water was added, extracted with EA, dried, and frozen to obtain the product 2-methoxy-5-amino - 4.1 g of 4-methylpyrimidine, GC: 98%, yield 74%.
Embodiment 2
[0021] The present embodiment is the preparation method of 2-methoxy-5-amino-4-methylpyrimidine, according to the following reaction formula:
[0022]
[0023] The specific experimental process is as follows:
[0024] Add 2-methoxyl-5-nitro-4-methyl-6-chloropyrimidine 8g (0.04mol, 1.0eq) into the autoclave, add methanol 80ml, nickel 0.4g, triethylamine 4g (0.04mol, 1.0eq), flow hydrogen at 30°C and 2.0MPa, stir for 5h, after the reaction is complete, filter, concentrate the filtrate, add 20ml of water, heat extraction with EA, dry, and freeze to obtain the product 2-methoxy-5-amino - 4.3 g of 4-methylpyrimidine, GC: 98%, yield 78%.
Embodiment 3
[0026] The present embodiment is the preparation method of 2-methoxy-5-amino-4-methylpyrimidine, according to the following reaction formula:
[0027]
[0028] The specific experimental process is as follows:
[0029] Add 2-methoxy-5-nitro-4-methyl-6-chloropyrimidine 8g (0.04mol, 1.0eq) in the autoclave, add acetonitrile 80ml, nickel 0.4g, triethylamine 4g (0.04mol, 1.0eq), flow hydrogen at 30°C and 2.0MPa, stir for 5h, after the reaction is complete, filter, concentrate the filtrate, add 20ml of water, heat extraction with EA, dry, and freeze to obtain the product 2-methoxy-5-amino - 3.9 g of 4-methylpyrimidine, GC: 98%, yield 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com