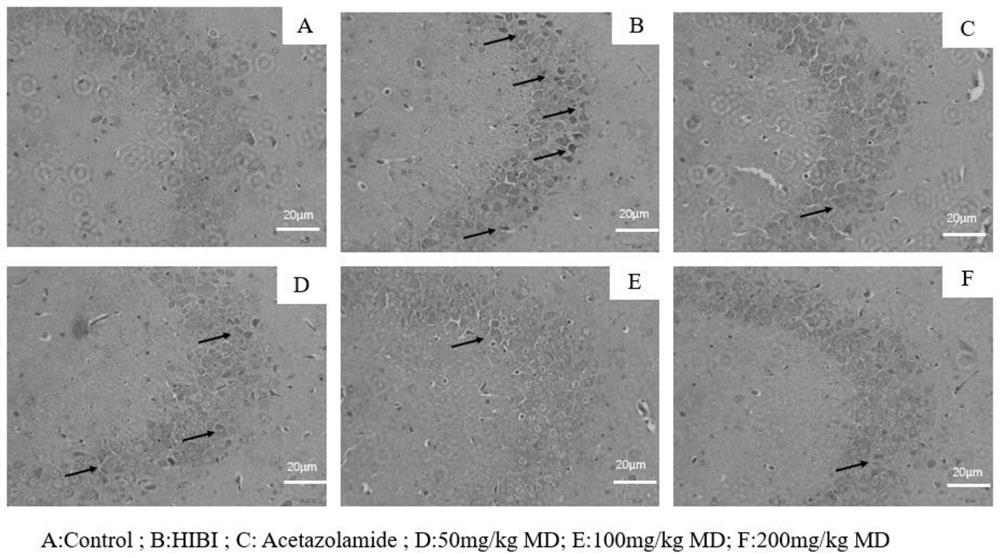
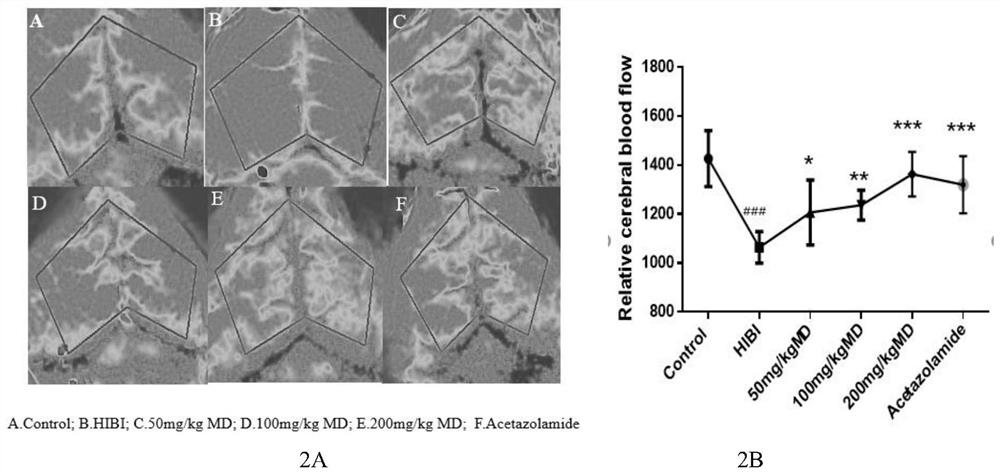
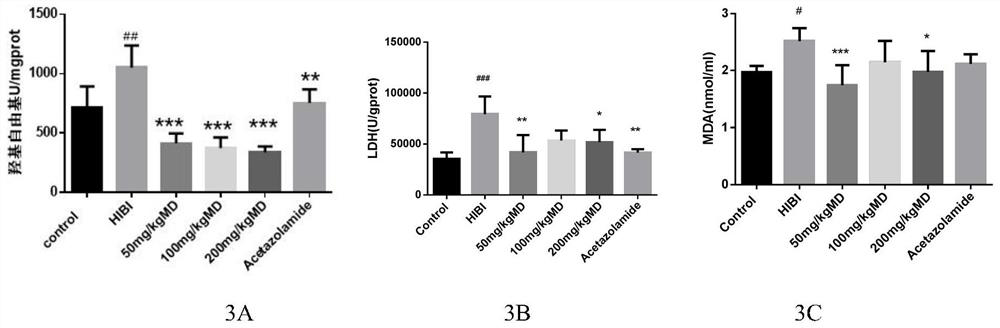
Meldonium as the application and medicine for preventing or treating acute brain injury at high altitude
A technology for acute brain injury and meldonium, applied in the field of biomedicine, can solve the problem of single category
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Meldonium was administered at a dose of 50 mg / kg.
[0109] The solid oral medicament of the present invention is composed of the following materials: 200 parts of meldonium, 10 parts of diluent, 10 parts of lubricant, 10 parts of binder, 10 parts of disintegrant, 10 parts of boiling mixture, 10 parts of sweetener, 2 parts of dye, 10 parts of wetting agent, 5000 parts of distilled water;
[0110] The above-mentioned solid oral medicine was administered continuously for three days, and the dosage was 8 mL / kg.
Embodiment 2
[0112] Meldonium was administered at a dose of 100 mg / kg.
[0113] The solid oral medicament of the present invention is composed of the following materials: 200 parts of meldonium, 10 parts of diluent, 10 parts of lubricant, 10 parts of binder, 10 parts of disintegrant, 10 parts of boiling mixture, 10 parts of sweetener, 2 parts of dye, 10 parts of wetting agent, 5000 parts of distilled water;
[0114] The above-mentioned solid oral medicine was administered continuously for three days, and the dosage was 8 mL / kg.
Embodiment 3
[0116] Meldonium was administered at a dose of 100 mg / kg.
[0117] The solid oral medicament of the present invention is composed of the following materials: 200 parts of meldonium, 10 parts of diluent, 10 parts of lubricant, 10 parts of binder, 10 parts of disintegrant, 10 parts of boiling mixture, 10 parts of sweetener, 2 parts of dye, 10 parts of wetting agent, 5000 parts of distilled water;
[0118] The above-mentioned solid oral medicine was administered continuously for three days, and the dosage was 8 mL / kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com