Method for determining content of impurity elements in uranium nitride solid sample
A technology of impurity elements and determination methods, applied in the field of chemical detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1 sample dissolved
[0056] Weigh 1.0g of sample, place the sample in a 150mL quartz beaker, add 5mL of concentrated nitric acid solution, heat and dissolve on a temperature-adjusted electric heating plate at 230°C to 260°C, after the sample is completely dissolved, evaporate it to a small amount at low temperature, and take Next, cool to room temperature.
[0057] 2 Separation of uranium matrix
[0058] Use 5.5mol / L nitric acid solution as the medium, wash the beaker three times, and transfer the sample to the quartz separatory funnel that has been added with 20mL uranium extraction reagent. Shake the separatory funnel for 30 seconds and let it stand for 10 minutes. Place the lower clear liquid in a well-balanced reversed-phase chromatography separation column. Use a 10mL quartz volumetric flask to receive the eluent to the mark, dilute to volume, and shake well for testing.
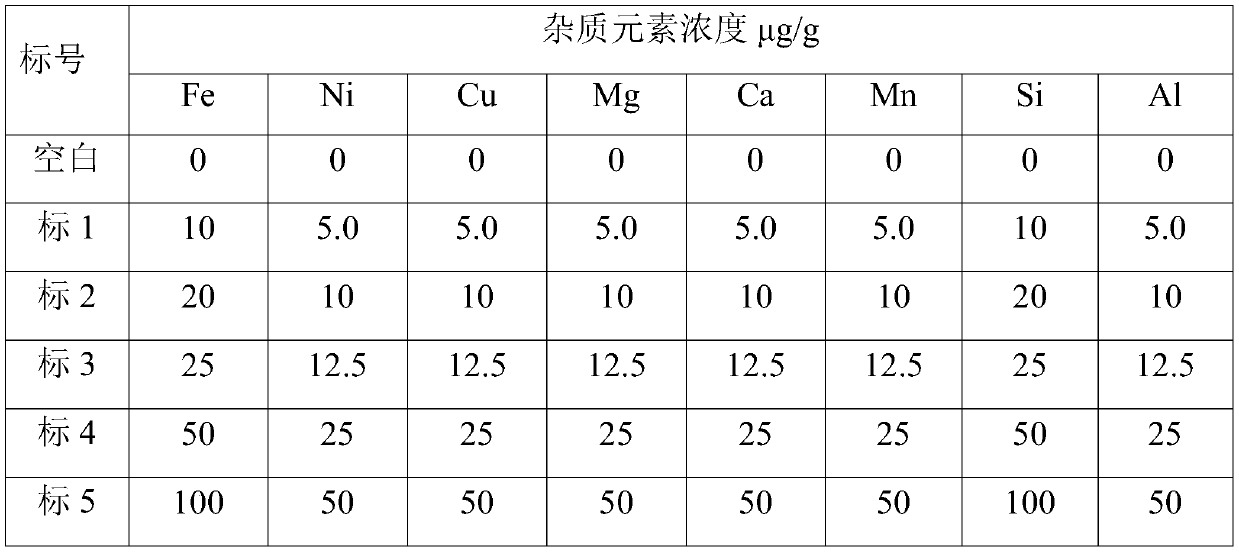
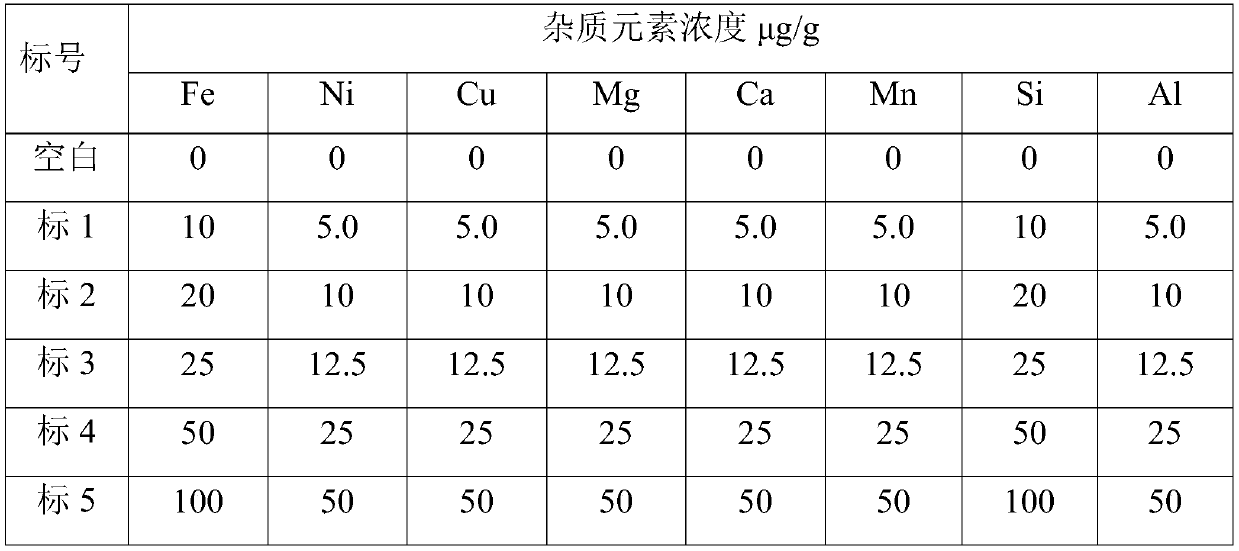
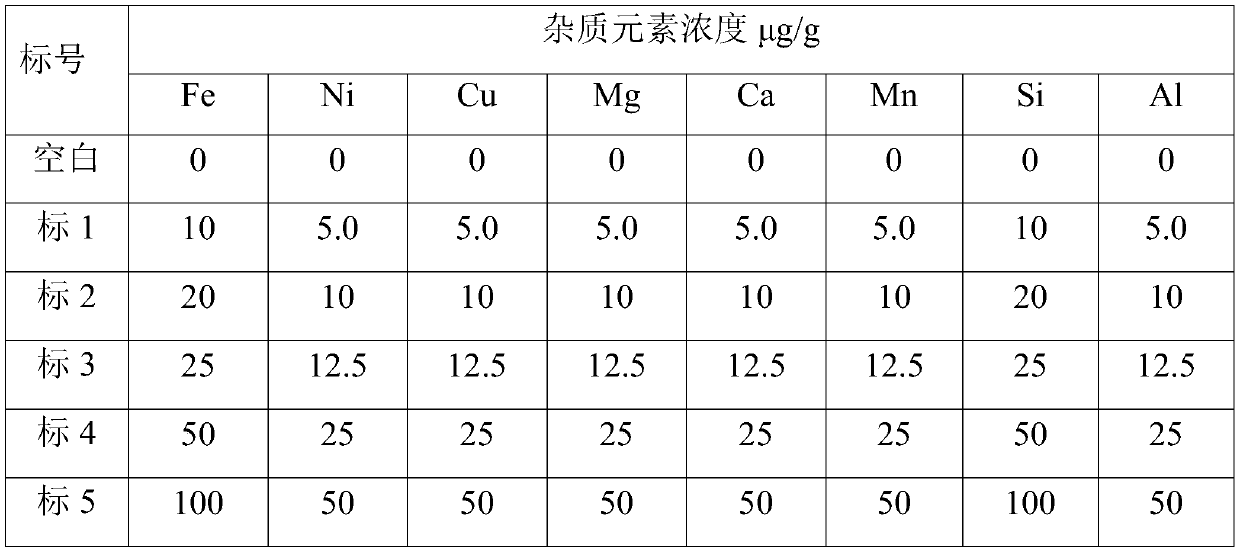
[0059] 3 Preparation of standard solution
[0060] Preliminary determination of each eleme...
Embodiment 2
[0084] 1 sample dissolved
[0085] Weigh 1.0g of sample, place the sample in a 150mL quartz beaker, add 7.5mL of concentrated nitric acid solution, heat and dissolve on a temperature-adjusting electric heating plate at 230°C to 260°C, and after the sample is completely dissolved, evaporate it to a small amount at low temperature. Remove and cool to room temperature.
[0086] 2 Separation of uranium matrix
[0087] Using 8mol / L nitric acid solution as the medium, wash the beaker three times, and transfer the sample to a quartz separatory funnel that has been added with 30mL uranium extraction reagent. Shake the separatory funnel for 30 seconds and let it stand for 10 minutes. Place the lower clear liquid in a well-balanced reversed-phase chromatography separation column. Use a 10mL quartz volumetric flask to receive the eluent to the mark, dilute to volume, and shake well for testing.
[0088] 3 Preparation of standard solution
[0089] Preliminary determination of each elem...
Embodiment 3
[0112] 1 sample dissolved
[0113] Weigh 1.0g of sample, place the sample in a 150mL quartz beaker, add 10mL of concentrated nitric acid solution, heat and dissolve on a temperature-adjusting electric heating plate at 230°C to 260°C, and after the sample is completely dissolved, evaporate it to a small amount at low temperature, and take Next, cool to room temperature.
[0114] 2 Separation of uranium matrix
[0115] Use 10mol / L nitric acid solution as the medium, wash the beaker three times, and transfer the sample to the quartz separatory funnel that has been added with 40mL uranium extraction reagent. Shake the separatory funnel for 30 seconds and let it stand for 10 minutes. Place the lower clear liquid in a well-balanced reversed-phase chromatography separation column. Use a 10mL quartz volumetric flask to receive the eluent to the mark, dilute to volume, and shake well for testing.
[0116] 3 Preparation of standard solution
[0117] Preliminary determination of each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com