Construction method and quality inspection method of ULC characteristic map of wind-resistant medicinal materials
A construction method and a technology of characteristic maps, applied in the field of construction of UPLC characteristic maps, can solve the problems of not being able to comprehensively evaluate the quality of windproof and not fully reflect the material characteristics of traditional Chinese medicine decoctions, and achieve the effect of overcoming the deviation of durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the construction method of the UPLC characteristic map of windproof medicinal material
[0048] The present embodiment provides a method for constructing a UPLC characteristic map of windproof medicinal materials, including the following:
[0049] 1. Instruments and reagents
[0050] Table 1. Instrument information table
[0051]
[0052]
[0053] Table 2. Reagent information table
[0054] Reagent name factory level batch number Chromatography Acetonitrile Shanghai Xingke HPLC grade 011470303 Chromatography methanol BCR HPLC grade 170320104001 water M MILLIPORE Synergy UV Ultra-pure water the day
[0055] Table 3. Reference substance information table
[0056]
[0057] Table 4. Information table of windproof medicinal materials
[0058]
[0059]
[0060] In the table, Cimicifolin glycosides and 5-O-methyl visamidol glycosides were determined according to the content determination meth...
Embodiment 2
[0191] Embodiment 2, a kind of quality detection method of windproof medicinal material
[0192] The present embodiment provides a quality detection method for windproof medicinal materials, comprising the following steps:
[0193] 1. The preparation of the solution and the chromatographic conditions are the same as those in "2. Chromatographic conditions and preparation of the test solution" in "Example 1".
[0194] 2. Determination Precision Absorb 1 μl of the test solution, inject it into the liquid chromatograph, and measure it.
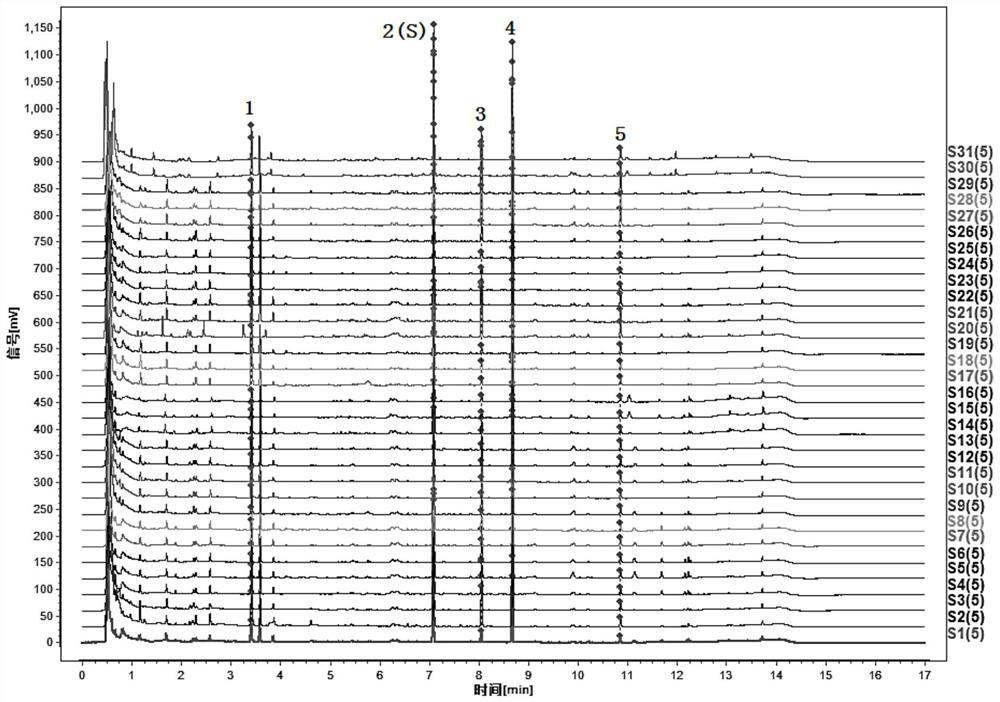
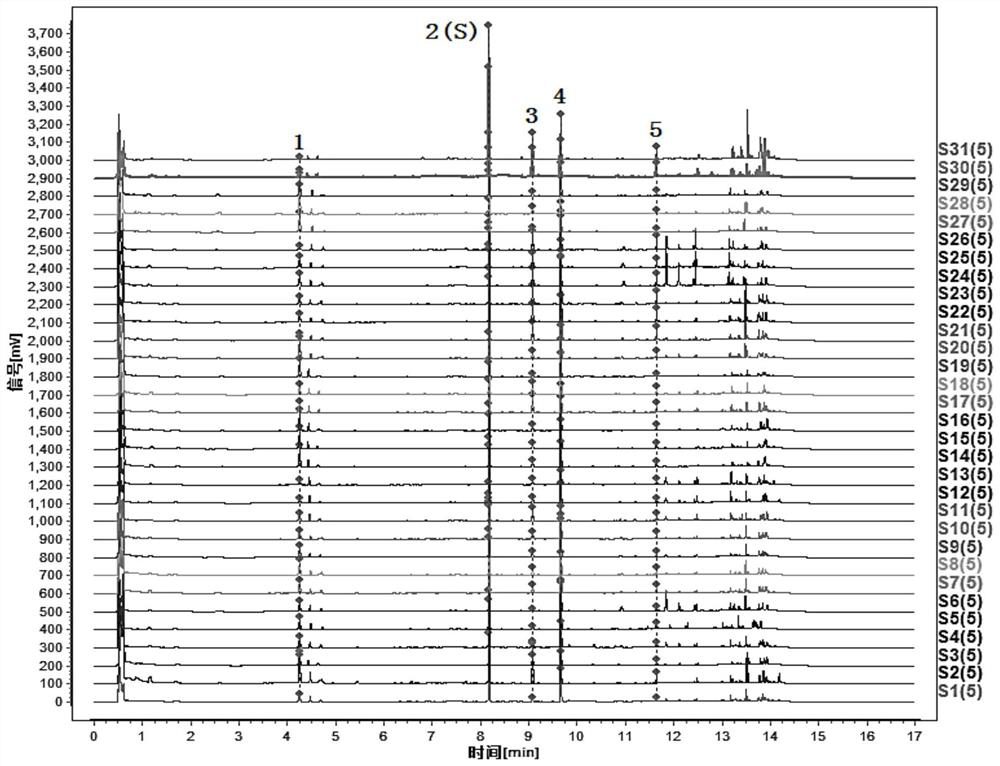
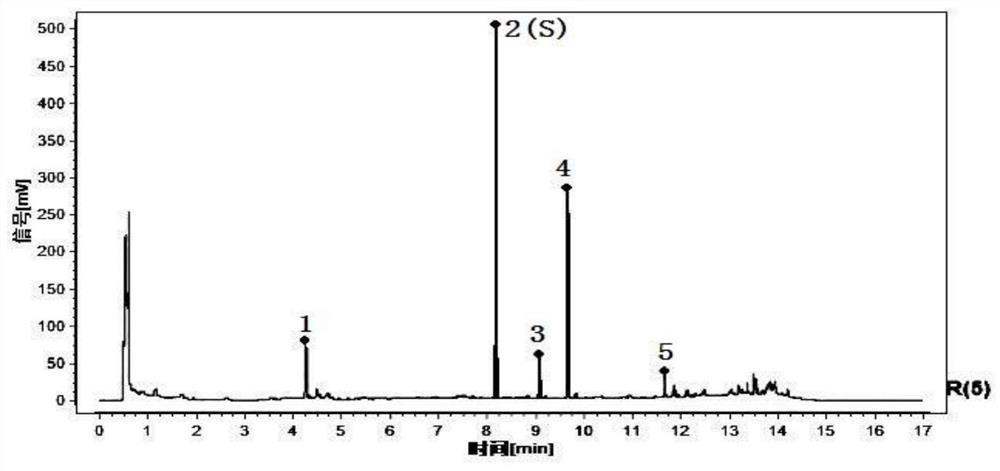
[0195] Compare the characteristic spectrum of the medicinal herbs to be tested with the control characteristic spectrum of the medicinal herbs: the sample to be tested can detect 5 characteristic peaks, which should correspond to the 5 characteristic peaks in the chromatogram of the reference substance of the control medicinal material, and the The peak corresponding to the reference peak is the S peak. Calculate the relative retention time of...
Embodiment 3
[0196] Embodiment 3, the identification of wild windproof and cultivated windproof medicinal materials
[0197] The present embodiment provides a method for identifying wild windbreaks and cultivated windbreaks, including the following:
[0198] 1. Test drug
[0199] Table 23. List of medicinal materials for windproofing
[0200] serial number Medicinal batch number Remark 1 YSFF01 wild 2 YSFF02 wild 2 YSFF03 wild 3 YSFF04 wild
[0201] 2. The preparation of the solution and the chromatographic conditions are the same as those in "2. Chromatographic conditions and preparation of the test solution" in "Example 1".
[0202] 3. Determination Precision Absorb 1 μl of the test solution, inject it into the liquid chromatograph, and measure it.
[0203] 4. Results
[0204] 5 characteristic peaks can be detected in 4 batches of wild parsnip samples, which correspond to the 5 characteristic peaks in the chromatogram of the referen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com