A kind of intrinsic self-healing zwitterionic hydrogel and preparation method thereof
A zwitterion and hydrogel technology, which is applied in the field of intrinsic self-repairing zwitterion hydrogel and its preparation, can solve the problems of high preparation cost, poor self-repair performance, and high cost, and achieve good biodegradability and preparation method The effect of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of zwitterion-functionalized polyasparagine derivatives
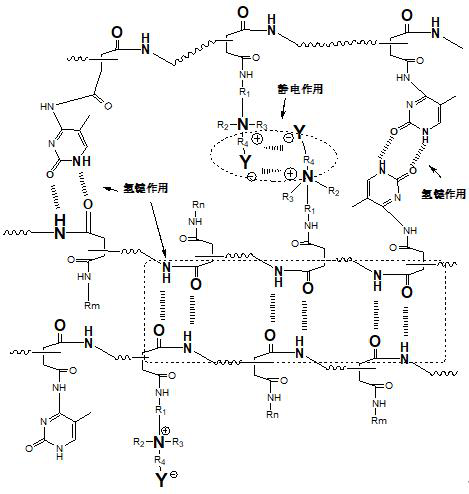
[0034]Dissolve 97g of polysuccinimide PSI (average molecular weight is about 7000g / mol) in 200g of DMF; add 30g of 5-methylcytosine, 20g of ethanolamine, 20g of N,N-dimethyl-1 , 3-propanediamine, react at 60 °C for 24 h for ring-opening reaction; then, add 24 g of 1,3-propane sultone, react at 38 °C for 8 h, and graft to the side of the polyasparagine derivative The tertiary amine group is subjected to quaternary amination reaction; after purification and drying, zwitterion-functionalized polyasparagine derivatives are obtained;
[0035] (2) Preparation of intrinsic self-healing zwitterionic hydrogels
[0036] Mix 100g of zwitterion-functionalized polyasparagine derivatives with 5000g of water, stir and dissolve evenly; add 5g of butanediamine, react at 66°C for 12h for cross-linking reaction, purify, and obtain intrinsic self-repairing zwitterions Hydrogels.
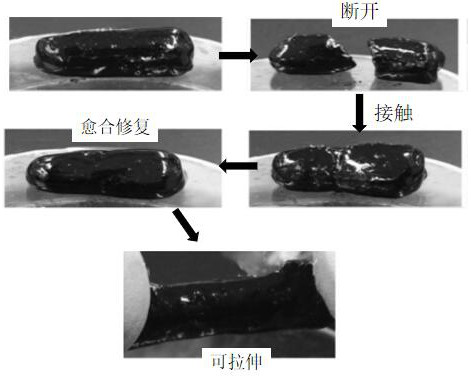
[0037] as attached figure 1 As ...
Embodiment 2
[0041] (1) Preparation of zwitterion-functionalized polyasparagine derivatives
[0042] Dissolve 97g of polysuccinimide PSI (average molecular weight is about 10000 g / mol) in 400g of DMSO; add 40g of 5-methylcytosine, 10g of isopropylamine, 15g of N,N-di Methyl-1,3-propanediamine was reacted at 40°C for 9h for ring-opening reaction; then, 18g of 1,3-propane sultone was added, reacted at 30°C for 18h, and grafted onto polyasparagine The pendant tertiary amine group of the derivative is subjected to quaternization reaction; after purification and drying, the zwitterion functionalized polyasparagine derivative is obtained;
[0043] (2) Preparation of intrinsic self-healing zwitterionic hydrogels
[0044] Mix 100g of zwitterion-functionalized polyasparagine derivatives with 500g of water, stir and dissolve evenly; add 6g of hexamethylenediamine, react at 70°C for 20h for cross-linking reaction, purify, and obtain intrinsic self-repairing zwitterions Hydrogels.
[0045] The pres...
Embodiment 3
[0048] (1) Preparation of zwitterion-functionalized polyasparagine derivatives
[0049] Dissolve 100 g of polysuccinimide PSI (average molecular weight is about 140,000 g / mol) in 100 g of DMF; add 10 g of 2-amino-4-hydroxy-6-methylpyrimidine, 10 g of arginine, 10 g of The N,N-dimethyl-1,3-propanediamine was reacted at 60°C for 24h for ring-opening reaction; then, 12g of 1,3-propane sultone was added, reacted at 40°C for 18h, and docked Branched to the pendant tertiary amine group of the polyasparagine derivative for quaternization reaction; after purification and drying, the zwitterion functionalized polyasparagine derivative is obtained;
[0050] (2) Preparation of intrinsic self-healing zwitterionic hydrogels
[0051] Mix 100g of zwitterion-functionalized polyasparagine derivatives with 1000g of water, stir and dissolve evenly; add 8g of triethylenetetramine, react at 60°C for 12h for cross-linking reaction, purify, and obtain intrinsic self-healing amphoteric ionic hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com