Method for rapidly evaluating in-vitro release of external preparation
An external preparation and rapid technology, applied in the direction of testing pharmaceutical preparations, instruments, surface/boundary effects, etc., can solve the problems of difficult animal skin acquisition and large individual differences, achieve effective in vitro release evaluation, eliminate individual differences, and the method is simple and easy line effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Selection of test equipment
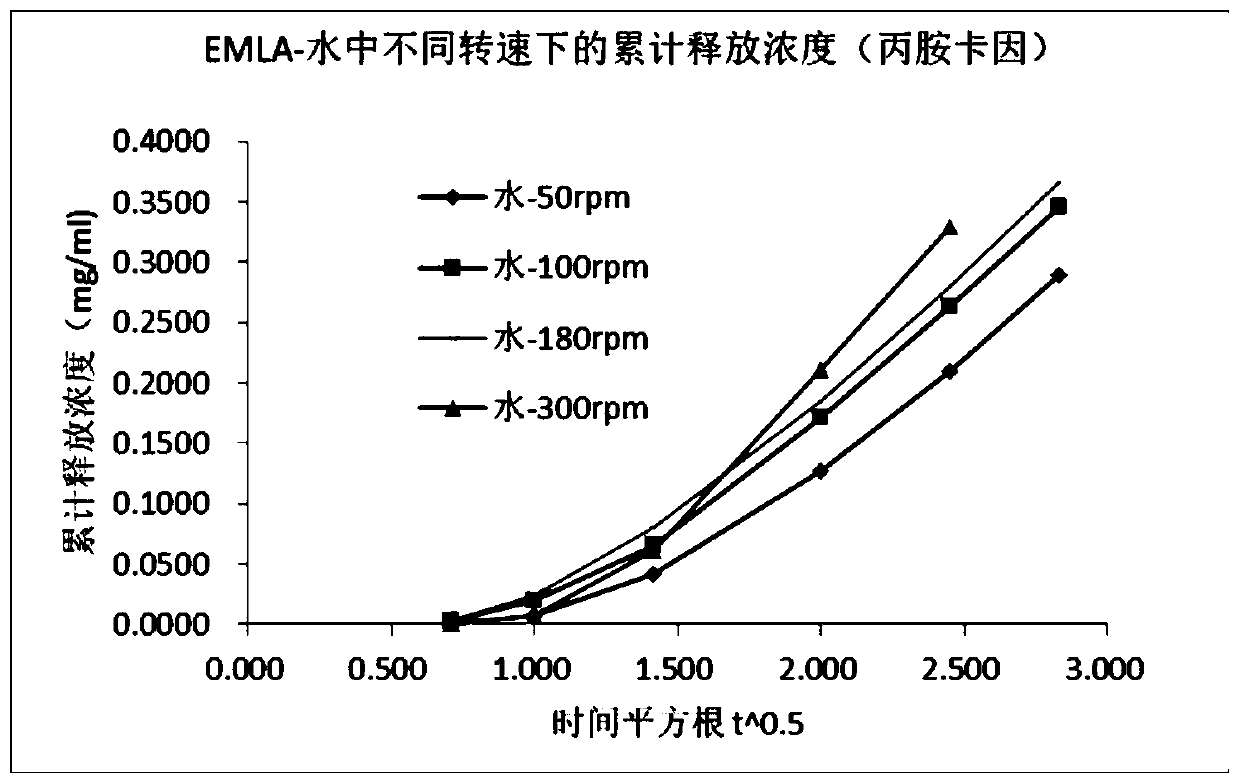
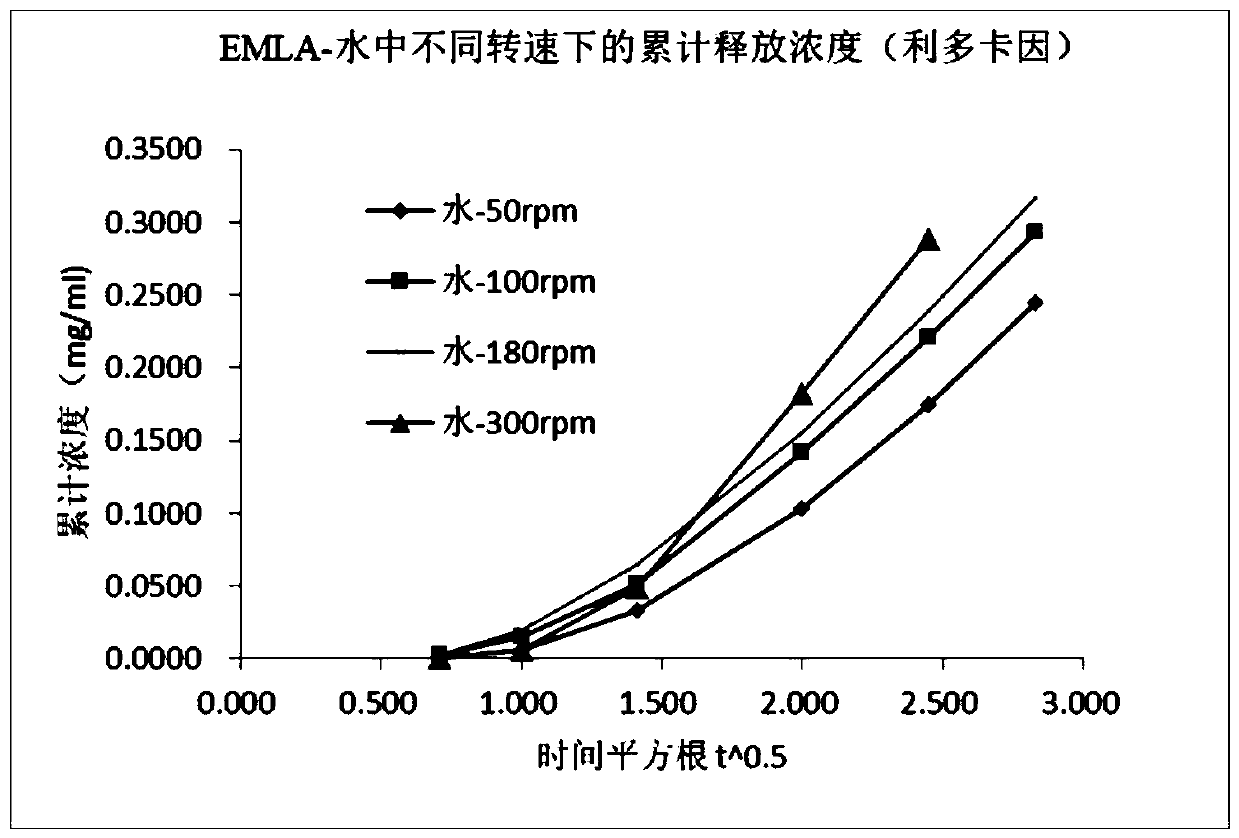
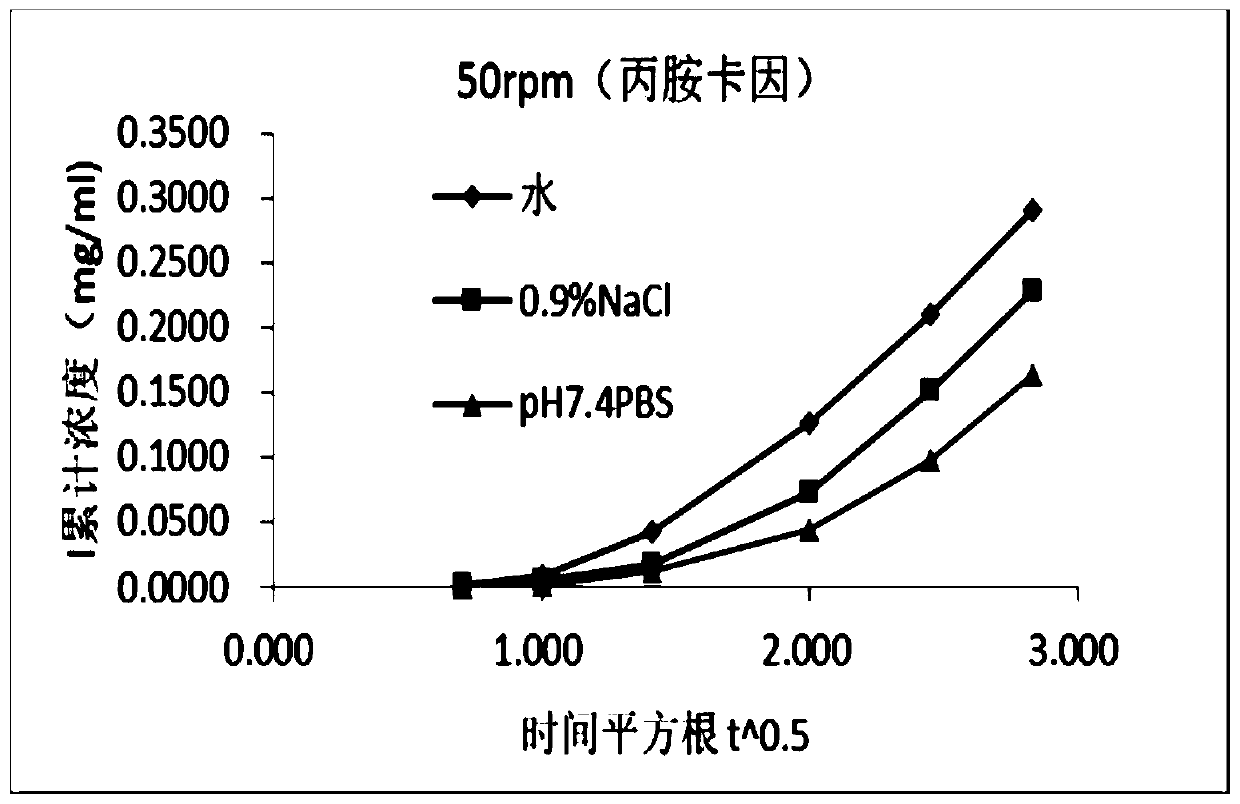
[0051] There are many measuring devices for in vitro release tests of semi-solid preparations, such as the fourth method of the United States Pharmacopoeia-FlowThrough Cell, Franz Diffusion cells, dynamic immersion cells, and improved semi-solid Accommodating method, the paddle-and-disk method of Chinese Pharmacopoeia. The Franz diffusion cell method is the most convenient and practical. This application is based on the Franz diffusion cell method for method development.
[0052] Diffusion Cell System Selected: Diffusion cell system with standard open cap ground glass like surface, 15mm diameter injection hole, 25mm overall system diameter.
Embodiment 2
[0054] Choice of artificial membrane
[0055] The Franz diffusion cell system needs to install an artificial membrane, which is produced by Merck Millipore. The Membrane artificial membrane is used as the artificial membrane in the in vitro release test system, and the diameter of the membrane is 25mm.
Embodiment 3
[0057] The choice of receiving liquid temperature
[0058] Referring to USP, when performing in vitro release test of semi-solid preparations for non-vaginal administration, the temperature of the receiving solution should be kept at 32°C±1°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com