Nasal hepatitis b vaccine composition and method for producing same
A vaccine composition, hepatitis B technology, applied in the direction of biochemical equipment and methods, drug combinations, microorganisms, etc., can solve the problems of incomplete success, non-existence, etc., and achieve the effect of low side effects
Pending Publication Date: 2020-06-26
TOKO YAKUHIN IND CO LTD +4
View PDF8 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, in its method of administration, it needs to be used in conjunction with subcutaneous vaccination to obtain an adequate immune response, i.e. it is a two-cycle vaccination, not a complete vaccine for nasal mucosal administration
[0007] As described above, as a next-generation hepatitis B vaccine, a nasal vaccine formulation that is widely used as an HBV vaccine for treatment and prevention is expected, but has not yet been completely successful
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
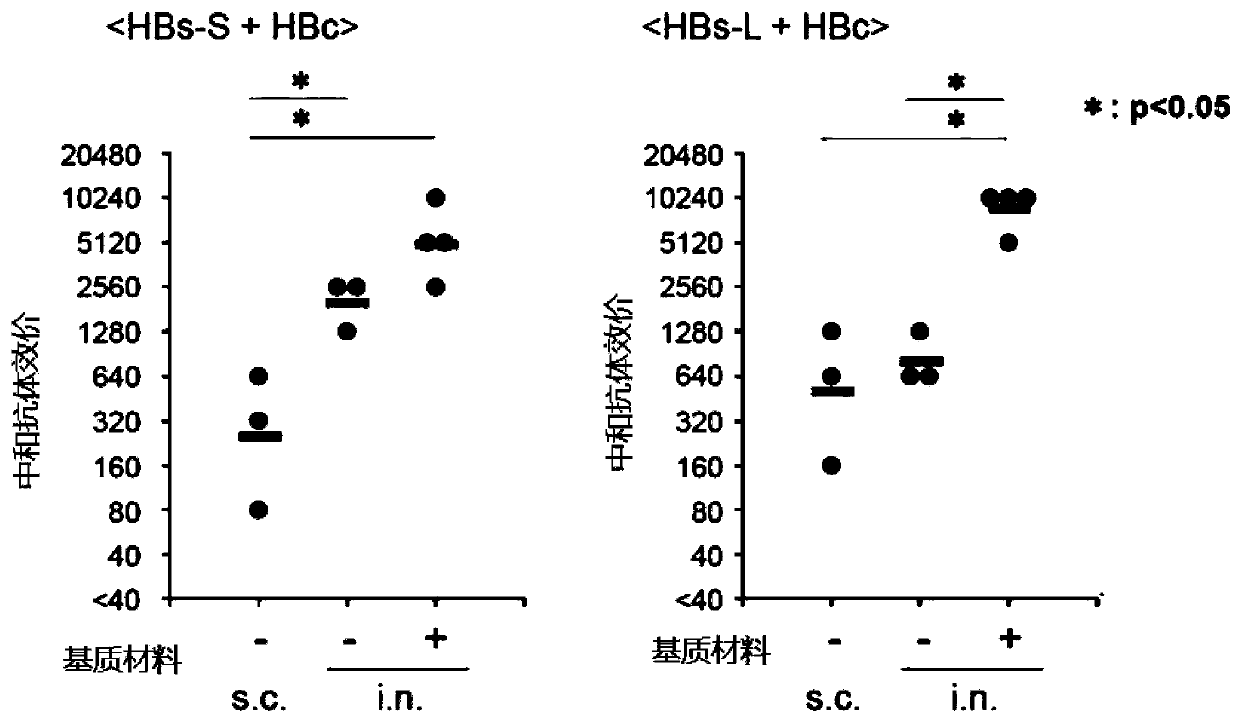
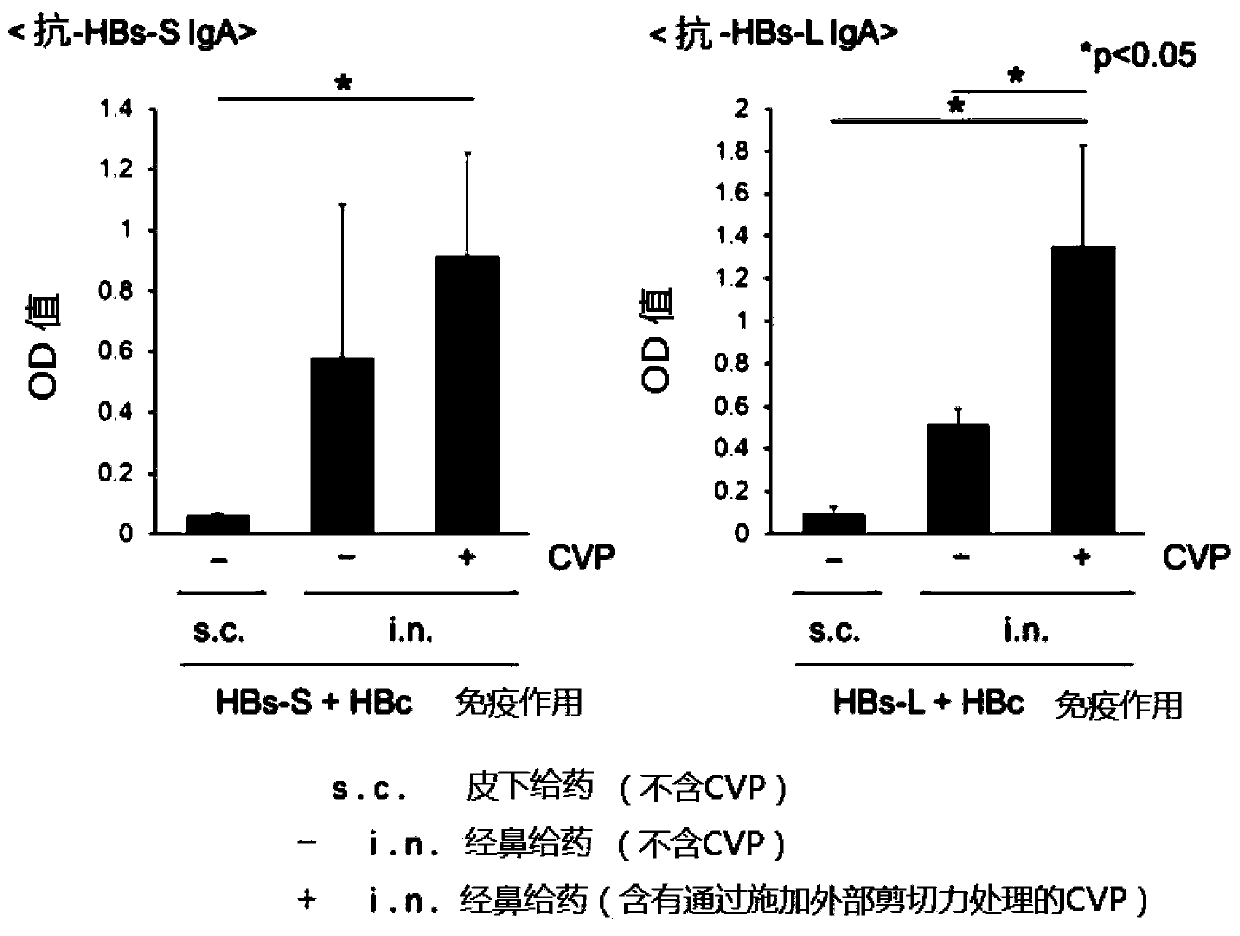
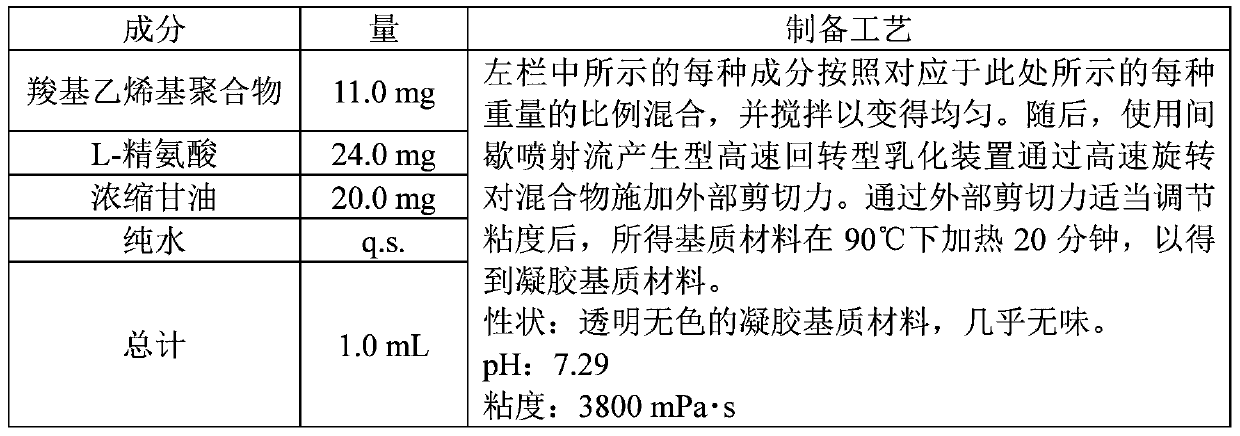
Embodiment 1
[0064]
[0065]
Embodiment 2
[0067]
[0068] Nasal hepatitis B vaccine compositions not containing gel matrix material were prepared as shown in the table below.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
The present invention relates to a hepatitis B vaccine composition for nasal mucosa spraying administration, characterized by comprising a hepatitis B antigen and a gel base containing a carboxyl vinyl polymer, and by being applicable to hepatitis B prevention and treatment.
Description
technical field [0001] The invention relates to a hepatitis B vaccine composition and its technology for nasal mucosal spray administration to prevent and treat hepatitis B. Background technique [0002] Hepatitis B is hepatitis caused by infection with the hepatitis B virus (HBV), which is transmitted through blood or body fluids. Persistent infection of liver cells by HBV can cause chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. [0003] Chronic hepatitis B (CHB) is currently treated mainly by using interferon agents (IFN) or nucleoside analog agents (NA) as first-line therapy. In IFN therapy, some effective examples have been reported, which increase immunity to effectively maintain the growth suppression of the virus, but in general, IFN therapy has a low HBV clearance rate and strong side effects, which is a big problem. On the other hand, NA therapy has a high HBV clearance rate of about 95%, but the therapeutic effect is temporary and cannot bring ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K39/00A61K9/12A61K39/29A61K47/32A61P31/20A61P37/04
CPCA61K47/32A61P31/20A61P37/04A61K39/12A61K2039/543C12N2730/10134A61K9/0043A61K47/183A61K47/10A61K39/292A61K9/124A61K9/0078
Inventor 上下泰造宮崎隆小原道法真田崇弘日浅阳一吉田理小原恭子长谷川秀树
Owner TOKO YAKUHIN IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com