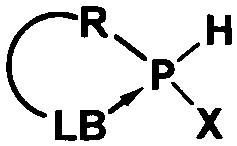
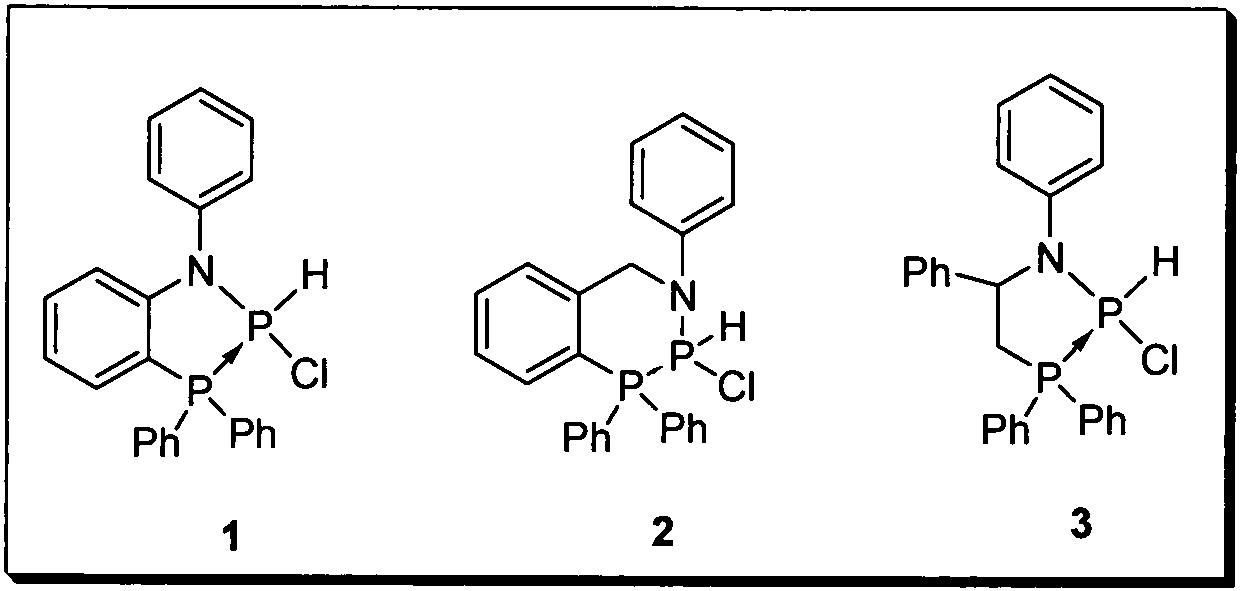
Preparation of nucleophilic phosphorene compound
A technology of nucleophilic phosphorene and compound, which is applied in the field of preparation of nucleophilic phosphorene compounds, and can solve the problems that phosphorene cannot be synthesized chemically and has poor reaction selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Precursor compound 1 (42 mg, 0.1 mmol) and crown ether 12-crown-4 (20 μl, 0.12 mmol) were placed in a Shrek bottle, 1.5 ml of diethyl ether was added, and freshly prepared diisopropyl Lithium amide was stirred at low temperature for 2 hours, and the solvent was dried with a vacuum pump. The crude product was washed with hexane (1ml*3), and then recrystallized with ether solvent at low temperature to obtain 28 mg of the target product with a yield of 73%.
Embodiment 2
[0012] Precursor compound 2 (43 mg, 0.1 mmol) and crown ether 18-crown-6 (32 mg, 0.12 mmol) were placed in a Shrek bottle, 2.0 ml of ether was added, and potassium tert-butoxide (13 mg, 0.12mmol), stirred at low temperature for 2h, dried the solvent with a vacuum pump, washed the crude product with hexane (1ml*3), and recrystallized with ether solvent at low temperature to obtain 27mg of the target product with a yield of 69%.
Embodiment 3
[0014] Precursor compound 3 (45mg, 0.1mmol) and crown ether 15-crown-5 (24μl, 0.12mmol) were placed in a Shrek bottle, 1.5ml of ether was added, and bis-(trimethylsilyl) was added at -78°C Base) sodium amide (22mg, 0.12mmol), stirred at low temperature for 2h, dried the solvent with a vacuum pump, washed the crude product with hexane (1ml*3), and recrystallized with ether solvent at low temperature to obtain 27mg of the target product, with a yield of 66 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com