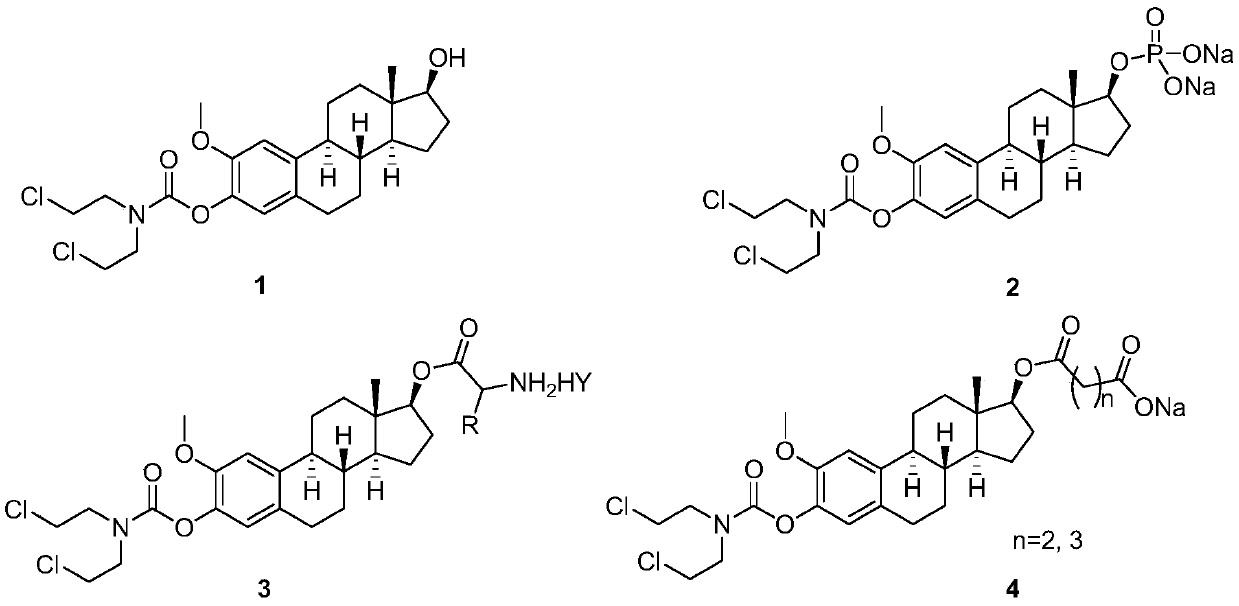
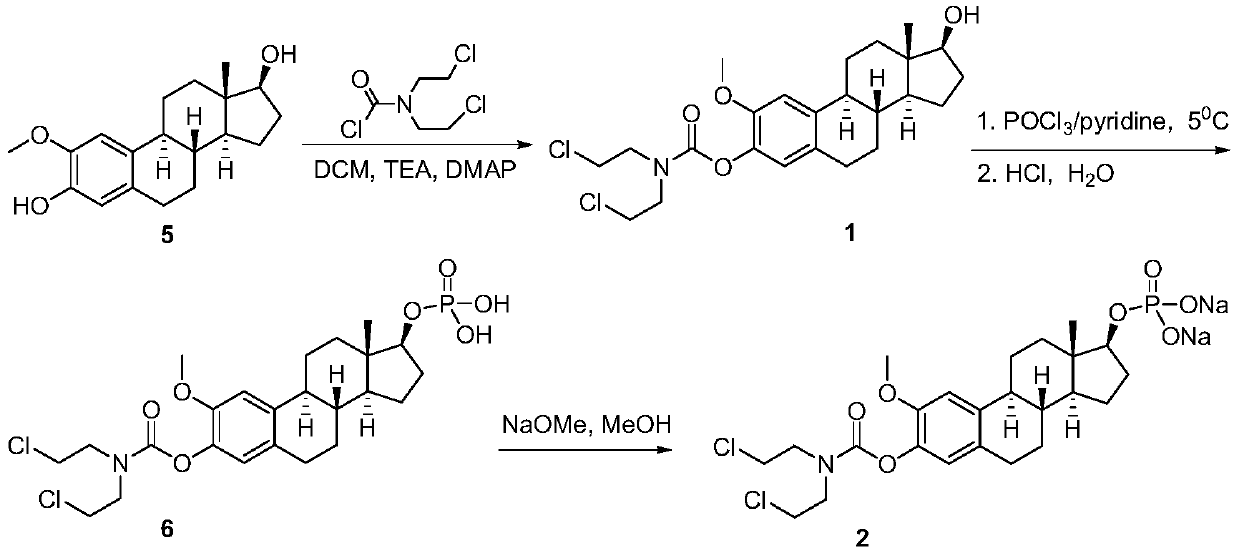
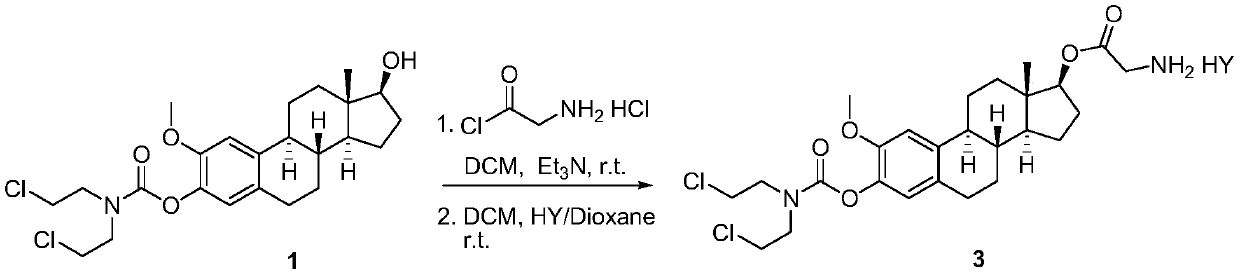
2-methoxyestramustine and derivative thereof, and preparation method and application thereof
A technology for methoxy estradiol and derivatives is applied in the field of preparation of a new compound 2-methoxy estramustine and its derivatives, which can solve the problems affecting the quality of life of patients, adverse reactions, insufficient targeting, and the like, Achieve the effect of improving bioavailability, less adverse side effects, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] The synthetic route of compound shown in formula 1 and formula 2 is as follows:
[0033]
[0034] The preparation method of the compound shown in the formula 1 comprises the following steps:
[0035]Dissolve 0.906g (3.0mmol) of 2-methoxy-1,3,5(10)-triene-3,17β-estradiol (5) in 30.0mL of dichloromethane, add 0.606g (6.0mmol ) triethylamine, and 0.037g (0.3mmol) 4-dimethylaminopyridine / DMAP, stirred at room temperature for 10 minutes; Add 5.0mL of dichloromethane slowly to the above reaction solution dropwise, the dropwise addition is completed within 10 minutes, continue to stir and react for 0.5 hours, then move the reaction solution to a water bath at 40°C for 2 hours, TLC detection shows that the reaction is complete , stop the reaction, cool the reaction solution to room temperature, add 50.0mL of distilled water, adjust the pH of the reaction solution between 5-6 with 10% hydrochloric acid solution under stirring, let the layers stand, separate the organic layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com