ACE inhibitory peptide P1 as well as application and preparation method thereof
A technology for inhibiting peptides and polypeptide sequences, which is applied in the field of ACE inhibitory peptide P1, can solve the problems of waste of fish skin resources, etc., and achieve the effects of good affinity, small toxic and side effects, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] In order to enable those skilled in the art to better understand the technical solution of the present invention, the present invention will be described in further detail below in conjunction with the accompanying drawings and specific embodiments.
[0038] The invention discloses an ACE inhibitory peptide P1, the amino acid sequence of which is: FNLRMQ.
[0039] The preparation method of ACE inhibitory peptide P1 comprises the following steps:
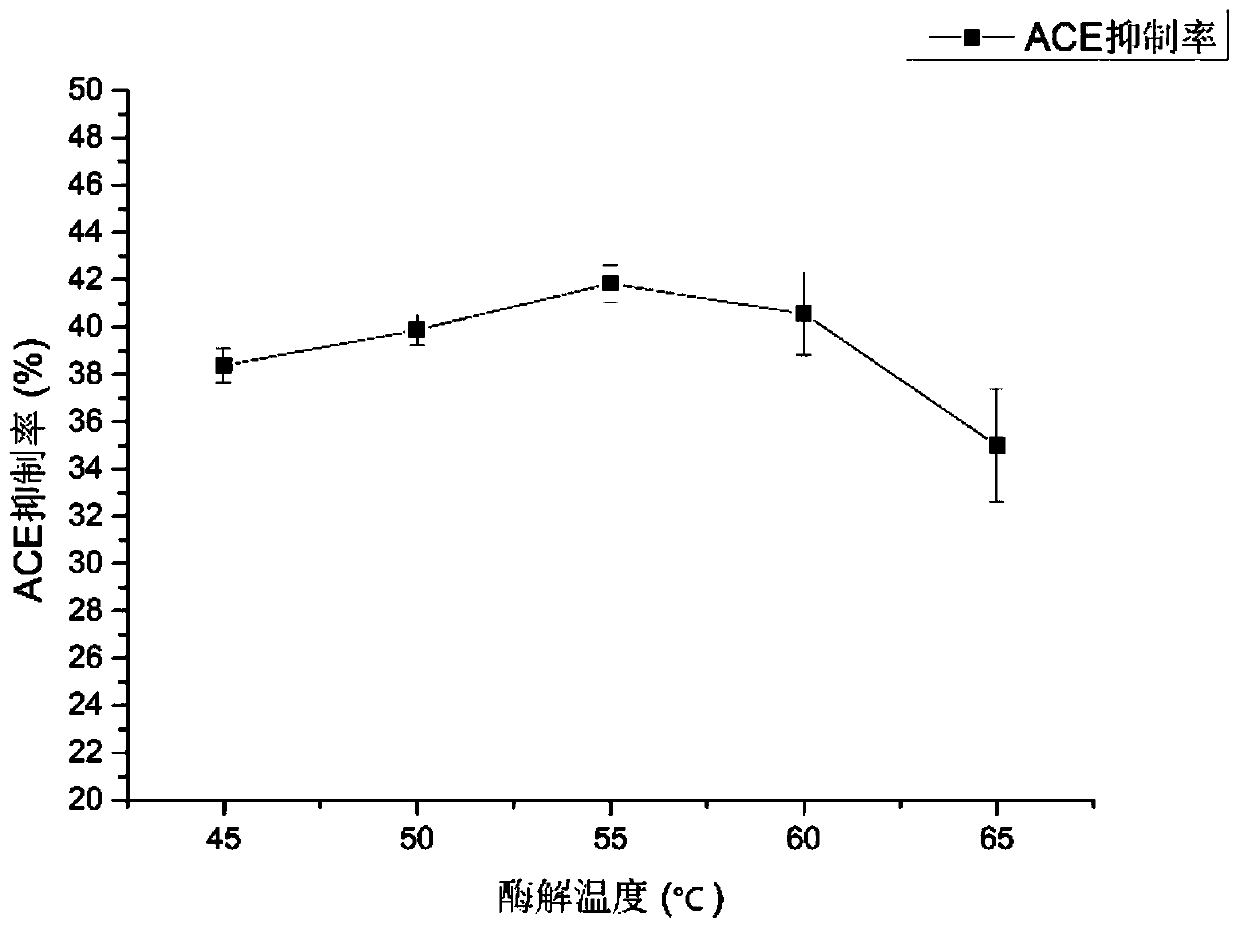
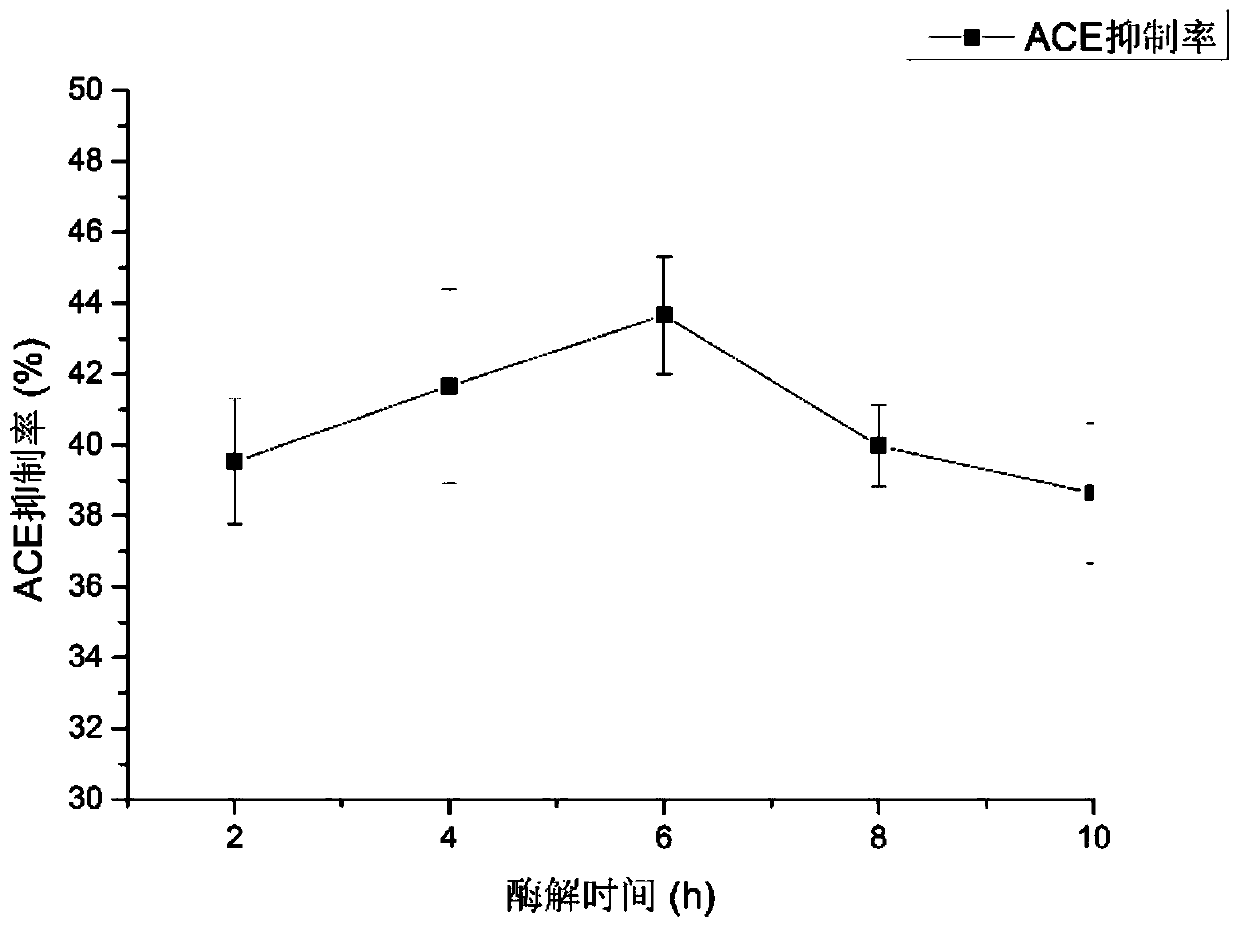
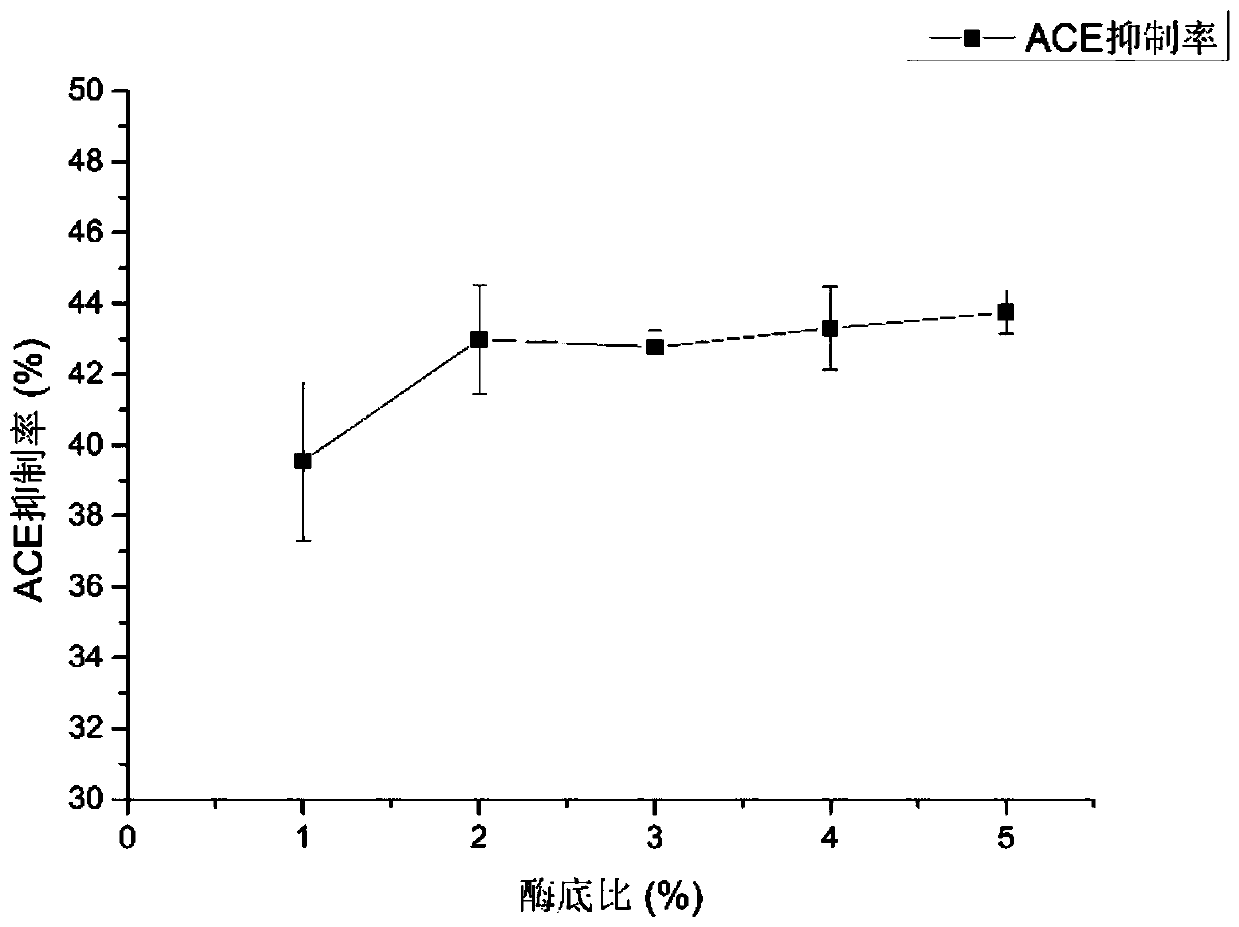
[0040] S1. Take fugu fish skin, add protease for enzymolysis, the enzymolysis temperature is controlled at 50°C-60°C, the enzyme-to-bottom ratio is 2%-4%, the pH is 11-12, and the enzymolysis time is 5h-7h. Preferably, the enzymolysis temperature is 55° C., the enzyme-to-substrate ratio is 2%, the pH is 12, and the enzymolysis time is 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com