Construction method and application of coronavirus antigen on the basis of mosaic strategy
A coronavirus and human coronavirus technology, applied in the direction of viral antigen components, viruses, viral peptides, etc., can solve the problems of ignoring the importance of T-cell immune response and no vaccine products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Analysis of Conserved CTL Epitopes on HCoVs Structural Proteins
[0081] In order to study the conserved T cell epitopes that exist on all known HCoVs, this embodiment first downloads a total of 3132 coronavirus genome sequences of all regions, all times, and all hosts from NCBI, VIPR and GISAID, including 928 HCoVs and 2204 other CoVs, and focused on the analysis of four structural proteins encoded by the HCoVs genome: S protein (spike-surface glycoprotein), E protein (small envelope protein), M protein (matrix protein) and N protein (nucleocapsid protein);
[0082] After using computer algorithms to remove repetitive sequences and sequences with poor sequencing quality, 534 S protein sequences, 485 M protein sequences, 410 N protein sequences and 478 E protein sequences were obtained, and the NetMHCpan-4.0 tool was used to predict the sequence of these proteins Potential cytotoxic T lymphocyte (CTL) epitopes present;
[0083] According to the phenotype freq...
Embodiment 2
[0086] Example 2 Design of Mosaic Antigen and Analysis of CTL Epitopes
[0087] In order to obtain artificial antigens that cover conserved CTL epitopes to the greatest extent, this example uses the Mosaic VaccineDesigner program to further analyze the S, M, N, and E protein sequences, and removes rare, low-frequency occurrences in natural epitopes (the number of occurrences is less than 3) After the epitope was identified, a series of mosaic antigens assembled from short peptides (composed of 9 amino acids) were obtained: S protein mosaic cocktail (SEQ ID NO: 1-4), M protein mosaic cocktail (SEQ ID NO: 5- 8), N protein mosaic cocktail (SEQ ID NO: 9-12) and E protein mosaic cocktail (SEQ ID NO: 13-16).
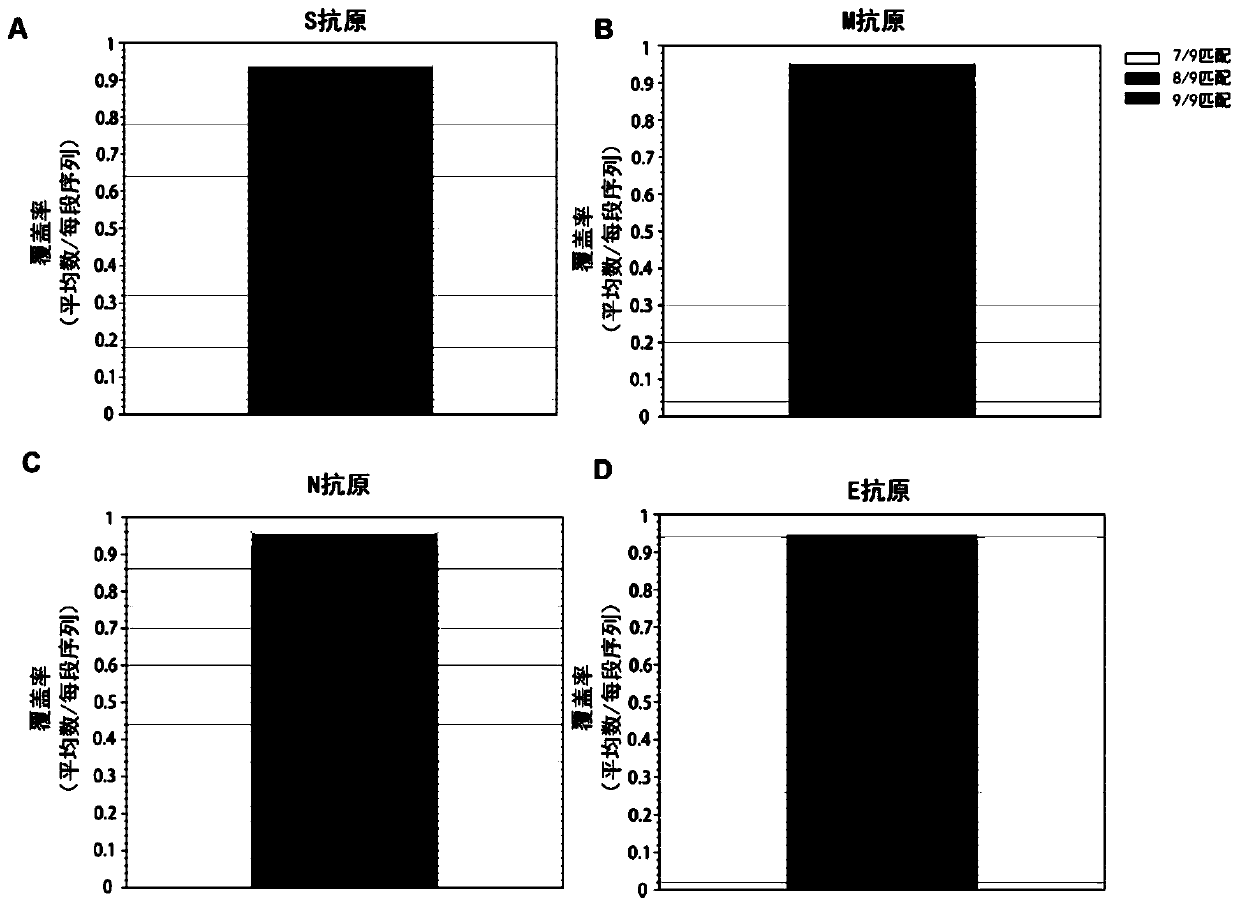
[0088] Analysis of the CTL epitopes on the four mosaic antigens revealed that most of the CTL epitopes on the natural S, M, N, E proteins were present on the mosaic antigens. Such as figure 1 As shown, 9 amino acids of 90% CTL epitopes on the mosaic antigen completely match ...
Embodiment 3
[0092] Phylogenetic Analysis of Example 3 Mosaic Antigens
[0093] This example explores the relationship between mosaic antigens and natural proteins. Such as image 3 As shown, 7 HCoVs form 4 clades, among which, A is the clade of HCoV-229E and HCoV-NL63, B is the clade of SARS-CoV and SARS-CoV-2, and C is the clade of HCoV-HKU1 and HCoV- The clade of OC43, D is the clade of MERS-CoV; the four mosaic antigens (stars) exist in each clade, indicating that the designed mosaic antigens widely cover all known HCoVs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com