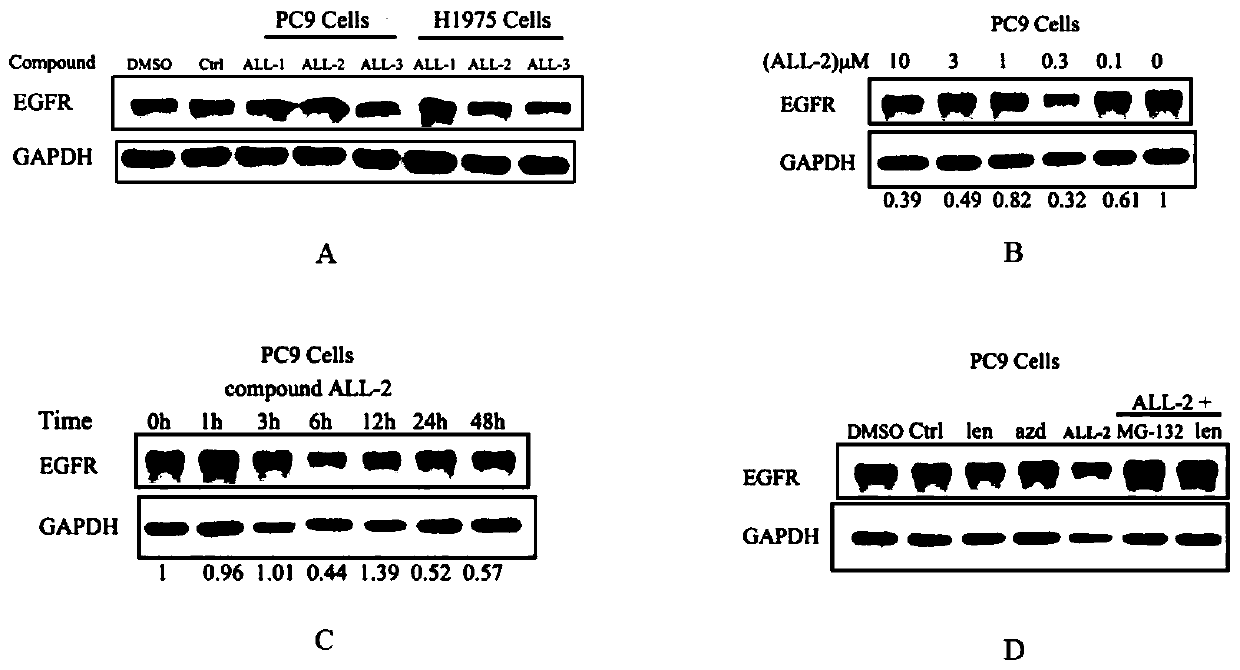
Lenalidomide-based small-molecular compound targeting to EGFR protein degradation, and preparation and application thereof
A compound and targeted technology, which can be used in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve the problems of limited inhibitory effect and drug resistance, and achieve good anti-tumor activity, good application prospects, and good EGFR. The effect of protein degradation levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of E3 Ligand Small Molecule Compound (Compound 6)
[0051] (1) Preparation of compound 2
[0052] Preparation of compound 2a:
[0053] Dissolve diethylene glycol (7.0g, 66.0mmol) in 10mL of dichloromethane solution, add triethylamine (13.3g, 132.2mmol), and add p-toluenesulfonyl chloride (15.1g, 79.1mmol) under ice-cooling. After the addition was complete, the reaction solution was stirred at room temperature for 12 h. Adjust the pH of the solution to 7 with 6 mol / L HCl, extract with dichloromethane (3×30 mL), combine the organic phases, and dry over anhydrous sodium sulfate. Column chromatography gave a yellow liquid product with a yield of 40%.
[0054] 1 H NMR (500MHz, Chloroform-d) δ7.75 (d, J = 8.4Hz, 2H), 7.31 (d, J = 8.0Hz, 2H), 4.19–4.09 (m, 2H), 3.62 (ddd, J = 12.1,5.4,4.1Hz,4H),3.48(dd,J=5.3,3.8Hz,2H),2.40(s,3H).
[0055] Preparation of compound 2b:
[0056] Dissolve triethylene glycol (5.0g, 33.3mmol) in 10mL of dichlorometha...
Embodiment 2
[0099] Embodiment 2: Preparation of PROTACs compound ALL-1
[0100]
[0101] Compound 6a (140mg, 0.26mmol) prepared in Example 1 was dissolved in 5mL N,N-dimethylformamide, compound 7 (141mg, 0.29mmol), N,N-diisopropylethylamine ( 101mg, 0.78mmol), heated at 90°C for 8h. Added 10 mL of water, extracted three times with 20 mL of dichloromethane, combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, and obtained a yellow solid by column chromatography with a yield of 53%. 1 H NMR (500MHz, CDCl 3 )δ9.74(s,1H),9.56(d,J=12.0Hz,1H),8.91(s,1H),8.78(d,J=11.5Hz,1H),8.37(dd,J=5.4,2.6 Hz,1H),8.15–7.99(m,1H),7.77(s,1H),7.70(dd,J=7.6,4.7Hz,1H),7.64(d,J=7.9Hz,1H),7.47–7.34 (m,3H),7.17(dd,J=5.3,3.3Hz,1H),6.72(s,1H),6.42(dd,J=16.9,1.8Hz,1H),5.69(dd,J=9.9,1.8 Hz,1H),5.13(dt,J=13.4,4.6Hz,1H),4.38(d,J=5.5Hz,2H),4.07(d,J=2.2Hz,2H),3.95(s,3H), 3.84(s,3H),3.75–3.51(m,8H),2.94(s,3H),2.83–2.65(m,5H),2.62(d,J=7.1Hz,4H),2.40–2.28(m, 4H),2.13–2.09(m,1H)p...
Embodiment 3
[0102] Embodiment 3: Preparation of PROTACs compound ALL-2
[0103]
[0104] Compound 6b (200mg, 0.35mmol) prepared in Example 1 was dissolved in 5mL N,N-diisopropylethylamine, and compound 7 (186mg, 0.38mmol), N,N-diisopropylethylamine (136mg, 1.05mmol), heated at 90°C for 8h. Add 10 mL of water, extract three times with 20 mL of dichloromethane, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and obtain a yellow solid by column chromatography with a yield of 62%. 1 H NMR (500MHz, CDCl 3 )δ9.79(s,1H),9.59(s,1H),8.98(d,J=17.9Hz,2H),8.38(d,J=5.3Hz,1H),8.12–8.01(m,1H), 7.79(s,1H),7.75–7.67(m,2H),7.44(t,J=7.8Hz,1H),7.41–7.35(m,1H),7.29–7.25(m,3H),7.18(d, J=5.3Hz, 1H), 6.76(s, 1H), 6.42(dd, J=16.6, 1.9Hz, 1H), 5.73–5.65(m, 1H), 5.16(dd, J=13.3, 5.2Hz, 1H ),4.39(s,2H),4.08(d,J=8.5Hz,2H),3.96(s,3H),3.85(s,3H),3.67(dt,J=5.3,2.6Hz,2H),3.61 –3.49(m,5H),3.42(dt,J=16.3,5.6Hz,5H),2.91(s,3H),2.83–2.71(m,2H),2.63(s,3H),2.52(s,2H ),2.29–2.26...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com