Method for treating inflammatory diseases
一种发炎性疾病、疾病的技术,应用在心血管系统疾病、抗炎剂、医药配方等方向,能够解决心血管疾病结果不一致等问题,达到高度生物兼容性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0068] Materials and Methods
[0069] Synthesis of DHLA-coated gold nanoclusters (FANC) and preparation of cultured cells
[0070] Human aortic endothelial cells (hereinafter referred to as HAEC) were cultured in endothelial cell growth containing 5% fetal bovine serum (FBS) and endothelial cell growth supplement (hereinafter referred to as complete medium; purchased from PromoCell, Heidelberg, Germany) Medium (MV). at 37°C with 5% CO 2 HAEC were cultured in complete medium in a humidified incubator with / 95% air and subcultured twice a week. The DHLA-coated gold nanoclusters used in this experiment were prepared based on precursor-induced etching of gold (Au) nanoparticles (NPs) (Lin et al., ACS Nano, 2009, 3(2), pp 395– 401). Briefly, first in toluene (tolune) by dissolving 0.8 ml of gold precursor (AuCl 3 , 7.5 mg / ml) was added to 1 ml of freshly prepared tetrabutylammonium borohydride (tetrabutylammonium borohydride, TBAB) (100 mmol / L DDAB solution) and 0.675 ml capri...
experiment example 1
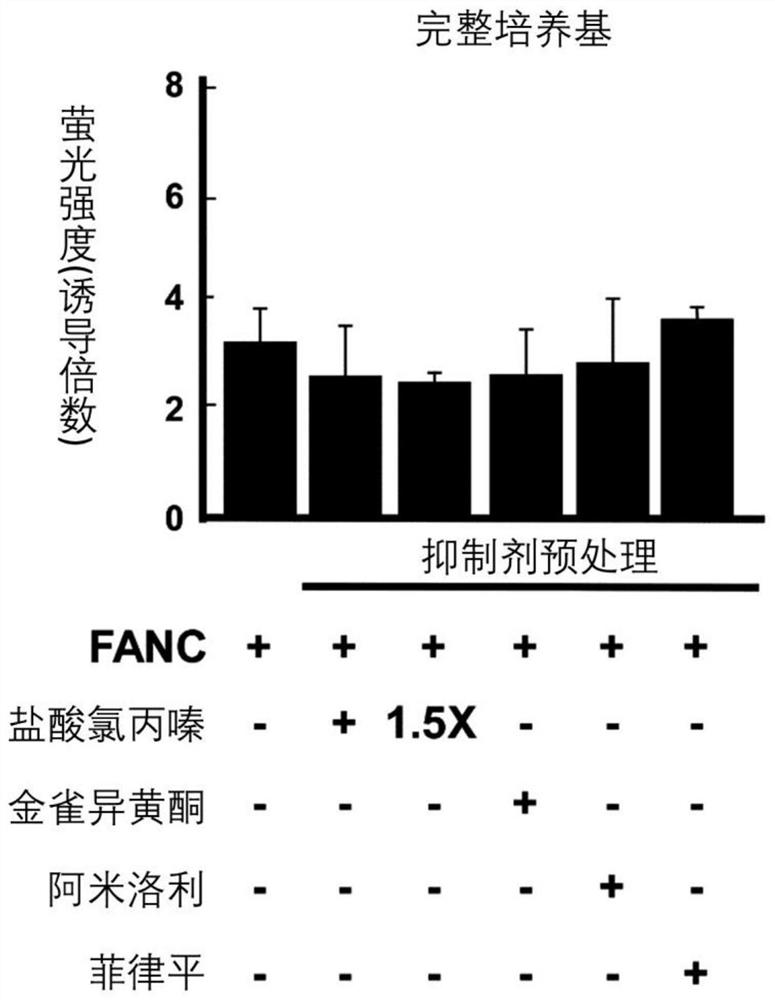
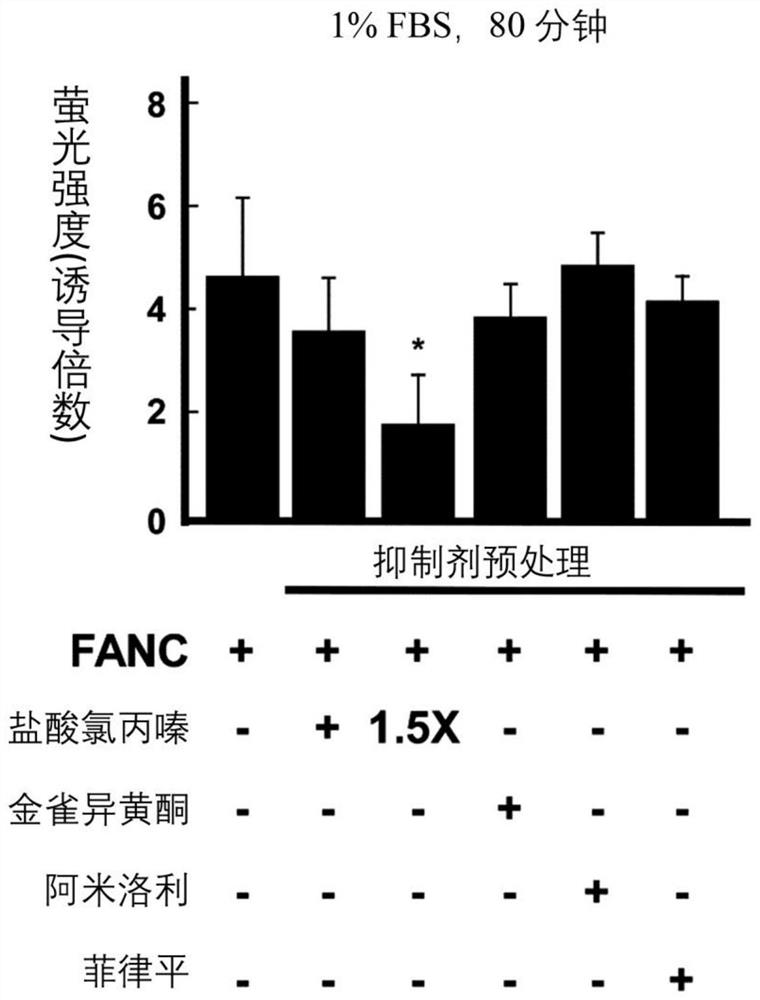
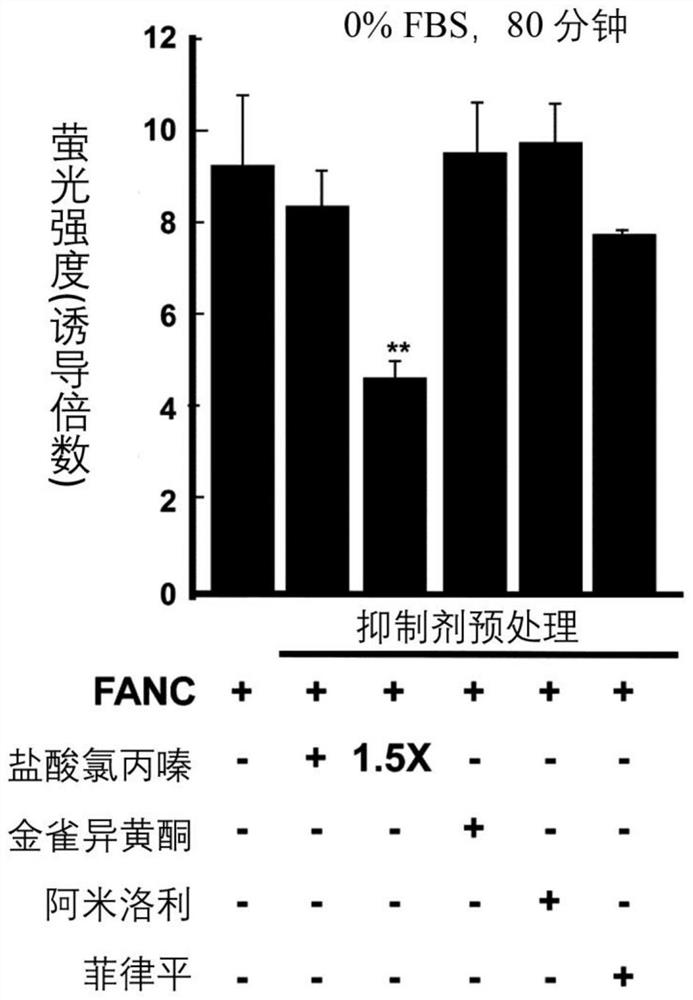
[0090] Experimental example 1: Effect of clathrin-mediated endocytosis on FANC internalization
[0091] After 45 minutes of inhibitor pretreatment (except for filipin which was treated for 30 minutes), FANC was added and incubation was continued for an additional 80 minutes. Next, the cells are passed through a flow cytometer. Figure 1A –1D). In complete medium, chlorpromazine hydrochloride and amiloride, which inhibit clathrin-dependent endocytosis and macropinocytosis, respectively, and inhibition of clathrin-non-clathrin endocytosis with minor effects on FANC internalization Agents (including genistein and filipin) ( Figure 1A ). However, in serum or serum-free medium, FANC internalization was blocked by chlorpromazine hydrochloride (compared to FANC control group, 1% FBS medium decreased by 60%, and serum-free medium decreased by 51%; Figure 1B and Figure 1C ). Confocal microscopy showed that chlorpromazine hydrochloride treatment could down-regulate the punctate...
experiment example 2
[0092] Experimental Example 2: Beneficial Effect of FANC on Inflammatory Response in Vitro
[0093] To examine cell markers that can be affected by FANC, cells maintained in complete medium supplemented with FANC were cultured for up to four weeks and assessed by real-time PCR at specific time points (days 21 and 28) . Transcripts of endothelial activity involved in angiogenesis, inflammation, coagulation, adhesion, growth, and vasodilation were examined. The results showed that the down-regulation of growth factors, adhesion molecules and coagulation factors was in a dose-dependent manner ( Figure 2A and Figure 2B ). It can be observed that compared with the control group (without FANC treatment), after 21 days of culture with FANC supplement (150nmol / L), the reduced molecules are VEGF, 88%; ICAM-1 (the diagram is ICAM): 64%; VCAM-1 (VCAM pictured): 74%; P-selectin: 88%; PAI-1: 64%; and vWF: 75% (Figure 2A; *: P Figure 2B ;*: P<0.05). In contrast, FANC had only minimal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com