Peripheral blood TCR marker for ovarian cancer as well as detection kit and application of peripheral blood TCR marker
A marker and ovarian cancer technology, applied in the field of genetic engineering, can solve the problems of less than 50% survival rate and inaccurate data, and achieve the effect of low labor cost, simple sampling, and reduced detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
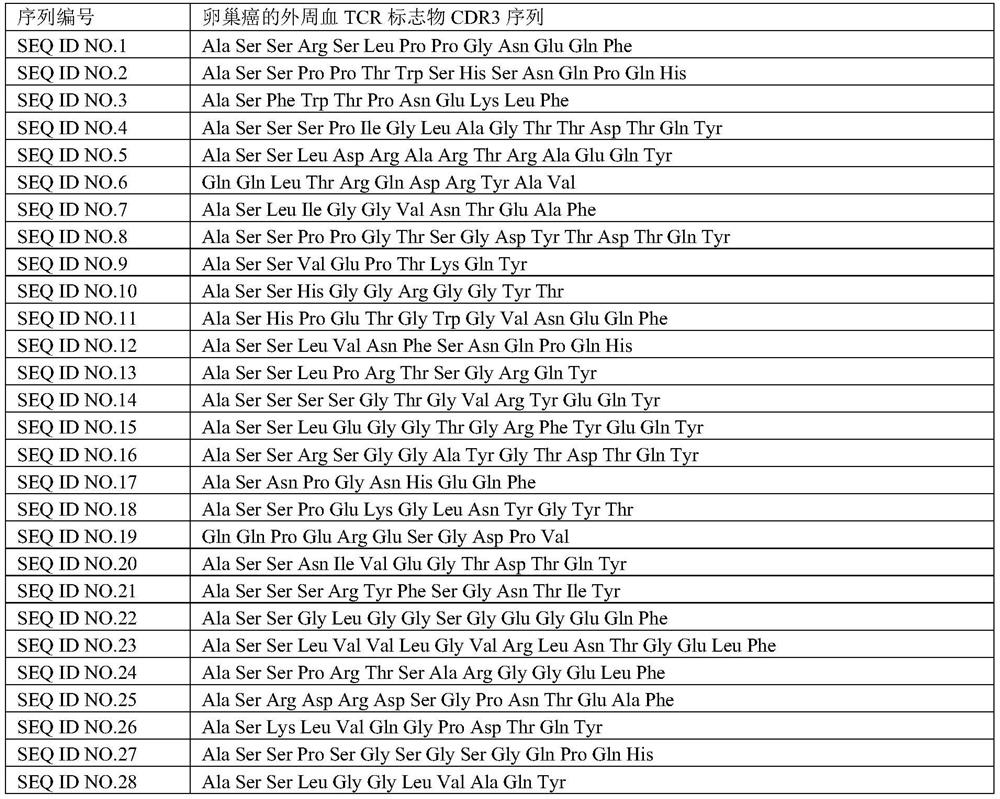
[0054] Example 1: Ovarian cancer TCR marker CDR3 sequence set was obtained by immunographic analysis
[0055] 1. Sampling and immune map analysis
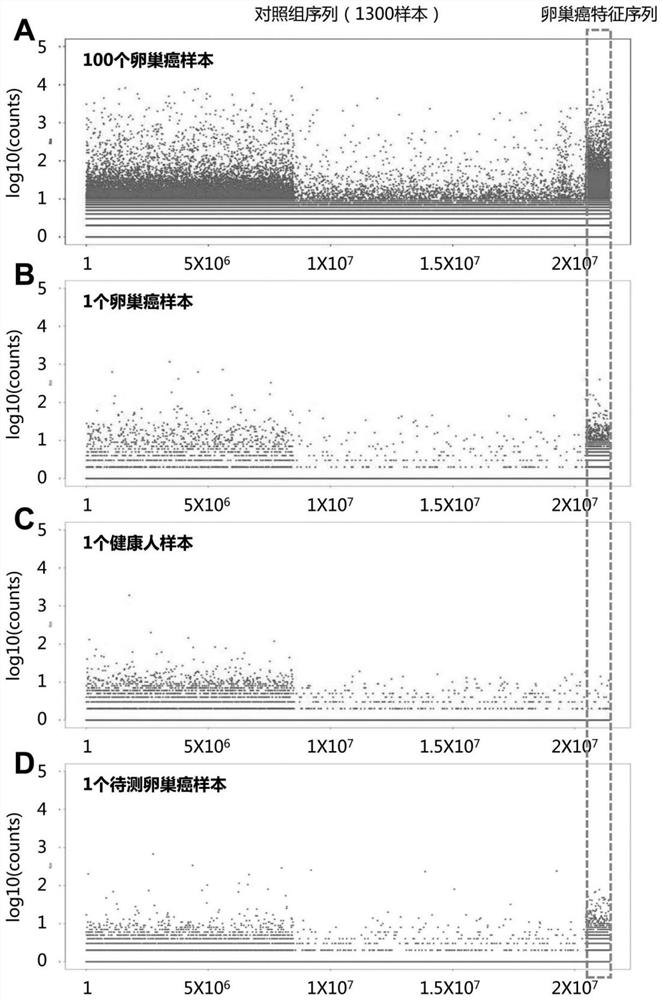
[0056] Collect 1301 control groups (including healthy people and patients with non-tumor diseases, 1300 people for model building, 1 healthy person for verification), 101 ovarian cancer patients (100 people for model building, 1 person for verification) and the peripheral blood of a subject with unknown health status (10mL per person), and the amino acid sequence of the TCR epitope 3 (CDR3) of the subject and the control group was obtained by high-throughput sequencing to ensure the functionality of each sample The total number of CDR3 sequences of TCR should not be less than 30,000;
[0057] 2. The CDR3 sequence of the TCR of each sample is randomly sampled without replacement, so that the total number of CDR3 sequences of each sample is 30,000. For any specific CDR3 sequence X, the number of repeated occurrences in the single-s...
Embodiment 2
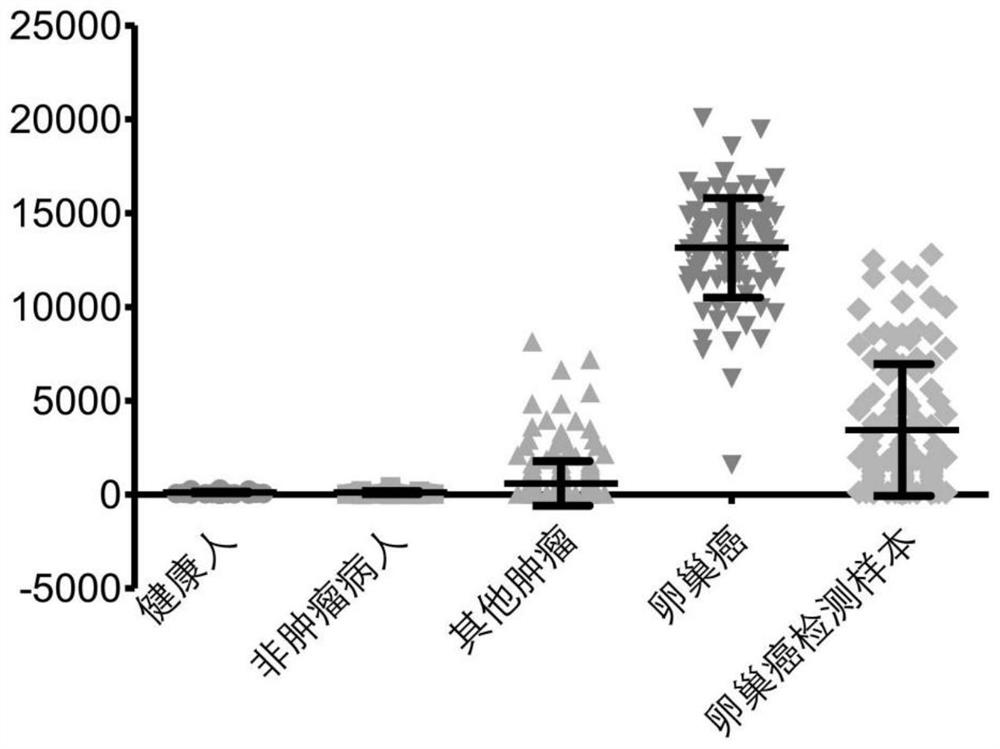
[0063] Example 2: Verification of the specificity of the ovarian cancer TCR marker CDR3 sequence set
[0064] 1. Sampling and immune map analysis
[0065] Peripheral blood (10 mL per person) was collected from 250 tumor patients without ovarian cancer and 107 subjects with unknown health status, and the epitope 3 (CDR3) amino acids of the TCR of the subjects and the control group were obtained by high-throughput sequencing Sequences, to ensure that the total number of CDR3 sequences of functional TCRs in each sample is not less than 30,000; random non-replacement sampling is performed on the CDR3 sequences of TCRs in each sample, so that the total number of CDR3 sequences in each sample is 30,000.
[0066] 2. Randomly select 100 healthy people and 45 non-tumor disease patients from the control group in Example 1.
[0067] 3. According to 100 healthy people, 45 non-tumor disease patients, and 100 ovarian cancer patients from Example 1, and 250 non-ovarian cancer tumor patients...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com