Tricyclohexyltin 5-bromo-2-furoate complex, and preparation method and application thereof
A technology of tricyclohexyl and complexes, applied in tin organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve the problems of application limitations, strong toxicity, etc., and achieve the effect of simple preparation method, low cost, and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
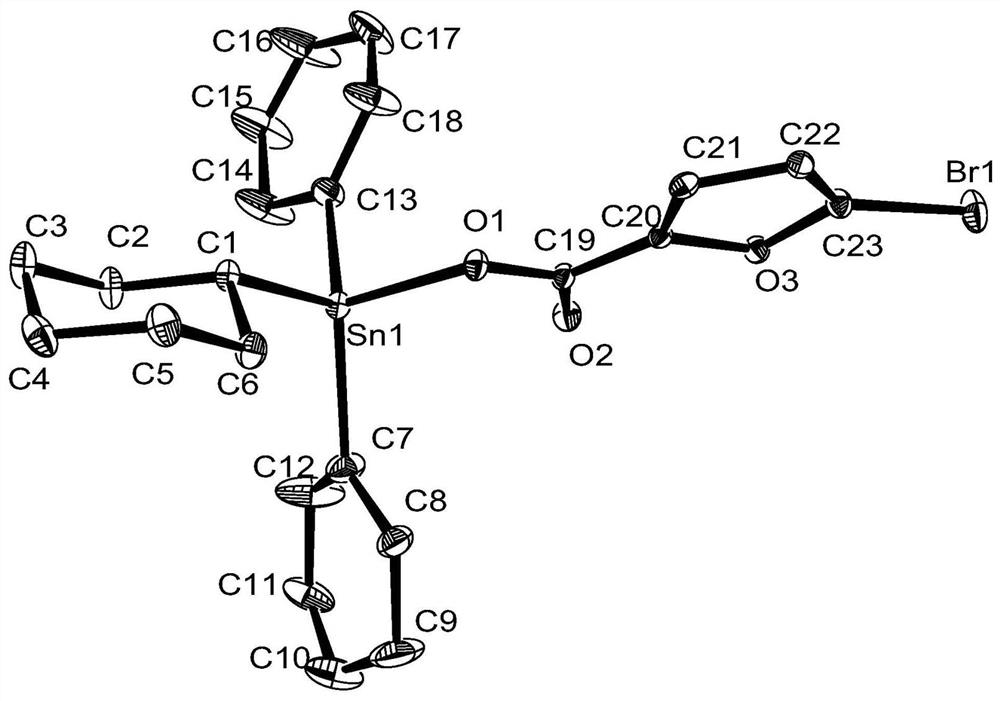
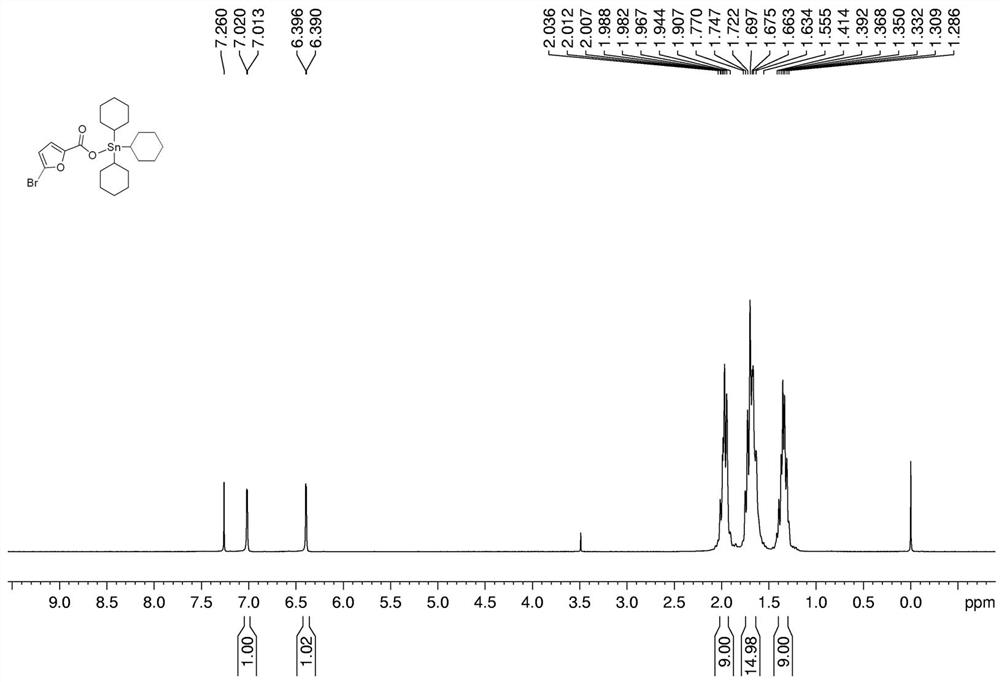
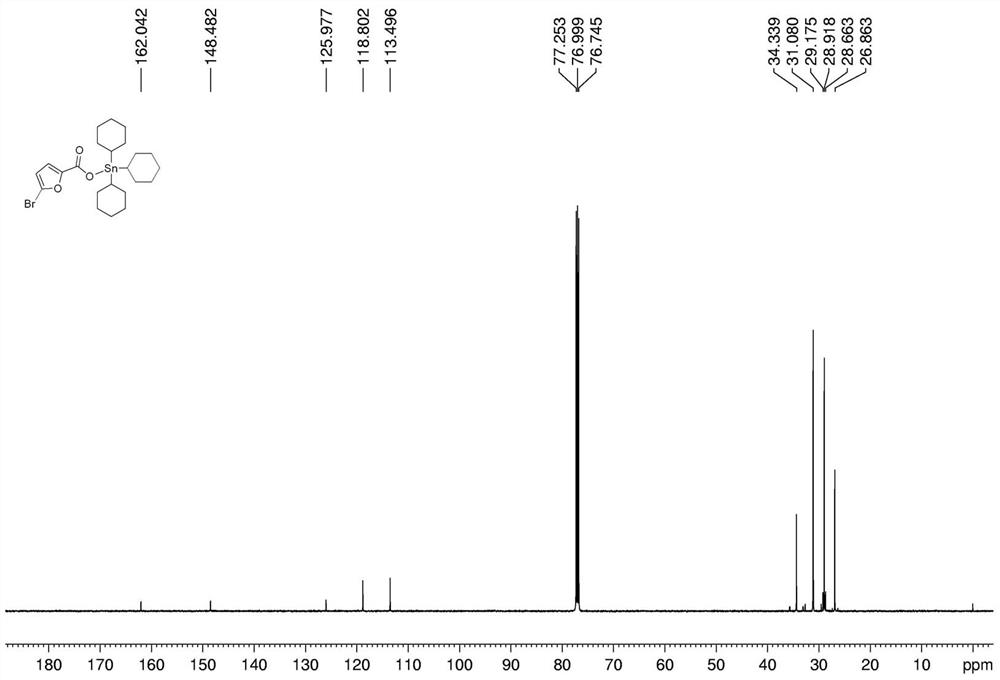
[0032] Preparation of tricyclohexyltin 5-bromo-2-furoate complex:
[0033] In a 250 mL round bottom flask, add 0.385 g (1 mmol) of tricyclohexyltin hydroxide, 0.1913 g (1 mmol) of 5-bromo-2-furoic acid, and 25 mL of solvent toluene in sequence, and install a Dean-Stark water separator Device, 112-120 ℃ heating reflux reaction for 6h. After the reaction, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a white solid, which is recrystallized from ethanol, which is tricyclohexyltin 5-bromo-2-furoate complex. Yield: 75%, melting point: 149-151°C.
[0034] Elemental analysis (C 23 h 35 BrO 3 Sn): Theoretical: C, 49.49; H, 6.32. Found: C, 49.46; H, 6.35.
[0035] IR(KBr, v / cm-1 ):3730.33(m), 2920.23(s), 2845.00(s), 2360.87(s), 2335.80(s), 1654.92(s), 1568.13(w), 1467.83(m), 1442.75(m), 1359.82(m ),1313.52(m), 1201.65(m), 1161.65(m), 1120.64(m), 1080.64(w), 1010.70(m),989.48(m), 916.19(m), 877.61(w), 802.39(m ), 773.76(m), 66...
Embodiment 2
[0041] Preparation of tricyclohexyltin 5-bromo-2-furoate complex:
[0042] In a 250 mL round-bottomed flask, add 0.3856 g (1.0 mmol) of tricyclohexyltin hydroxide, 0.2103 g (1.1 mmol) of 5-bromo-2-furoic acid, and 25 mL of solvent toluene in sequence, and install a Dean-Stark Water trap, reflux at 112-120 ℃ for 8 h. After the reaction, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a white solid, which is recrystallized from ethanol, which is tricyclohexyltin 5-bromo-2-furoate complex. Yield: 75%, melting point: 149-151°C.
[0043] Elemental analysis (C 23 h 35 BrO 3 Sn): Theoretical: C, 49.49; H, 6.32. Found: C, 49.46; H, 6.35.
[0044] IR(KBr, v / cm -1 ):3730.33(m), 2920.23(s), 2845.00(s), 2360.87(s), 2335.80(s), 1654.92(s), 1568.13(w), 1467.83(m), 1442.75(m), 1359.82(m ),1313.52(m), 1201.65(m), 1161.65(m), 1120.64(m), 1080.64(w), 1010.70(m),989.48(m), 916.19(m), 877.61(w), 802.39(m ), 773.76(m), 667.37(m), 597.93(...
Embodiment 3
[0050] Preparation of tricyclohexyltin 5-bromo-2-furoate complex:
[0051] In a 250 mL round-bottomed flask, add 0.3853 g (1.0 mmol) of tricyclohexyltin hydroxide, 0.2105 g (1.1 mmol) of 5-bromo-2-furoic acid, and 35 mL of solvent toluene in sequence, and install a Dean-Stark Water trap, reflux at 112-120 ℃ for 8 h. After the reaction, filter while it is hot, and remove the solvent from the filtrate with a rotary evaporator to obtain a white solid, which is recrystallized from ethanol, which is tricyclohexyltin 5-bromo-2-furoate complex. Yield: 75%, melting point: 149-151°C.
[0052] Elemental analysis (C 23 h 35 BrO 3 Sn): Theoretical: C, 49.49; H, 6.32. Found: C, 49.46; H, 6.35.
[0053] IR(KBr, v / cm -1 ):3730.33(m), 2920.23(s), 2845.00(s), 2360.87(s), 2335.80(s), 1654.92(s), 1568.13(w), 1467.83(m), 1442.75(m), 1359.82(m ),1313.52(m), 1201.65(m), 1161.65(m), 1120.64(m), 1080.64(w), 1010.70(m),989.48(m), 916.19(m), 877.61(w), 802.39(m ), 773.76(m), 667.37(m), 597.93(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com