Fangchinoline derivative with anticancer activity as well as preparation method and application thereof
A technology for fangchinoline base and anticancer activity is applied in the field of fangchinoline base derivatives and preparation, and can solve the problems of low bioavailability and wide application, and achieve the effects of low price of raw materials, novel structure and improved biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
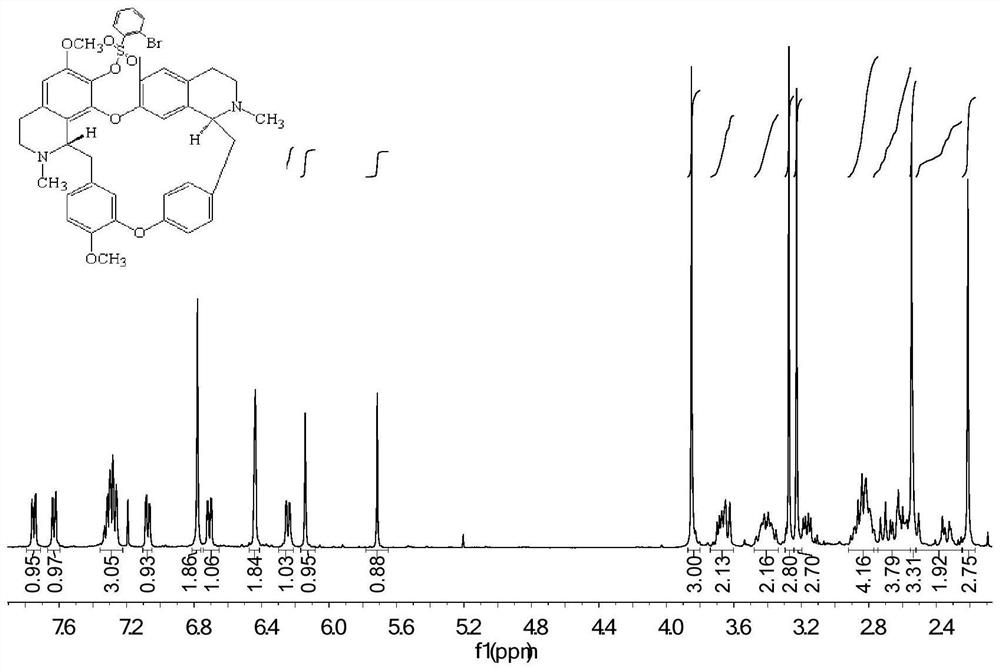
[0033] The preparation method of fangchinoline base derivatives with anticancer activity, first dissolve 100 mg fangchinoline base dTet (about 0.18 mmol) in 3 mL of dichloromethane, place it in a two-necked flask, and repeatedly inject argon into the flask Gas three times until the air is evacuated to fill the bottle with argon, and the temperature is controlled to 0 °C. Under magnetic stirring, 66.5 mg (about 0.72 mmol) of triethylamine dissolved in 2 mL of dichloromethane was added. After 1 hour of reaction, 50.7 mg (about 0.20 mmol) of 2-bromobenzenesulfonyl chloride was added, and the reaction was tracked by TLC. After the reaction, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was dichloromethane:methanol (120:1, 100:1, 80:1), separated and purified, and the white powder product 123.6 mg (about 0.149 mmol), the yield is 83%. After NMR and HRMS analysis, the obtained product is 7-O-(m-Br-benzenesulfonyl) fangchinoline base...
Embodiment 2
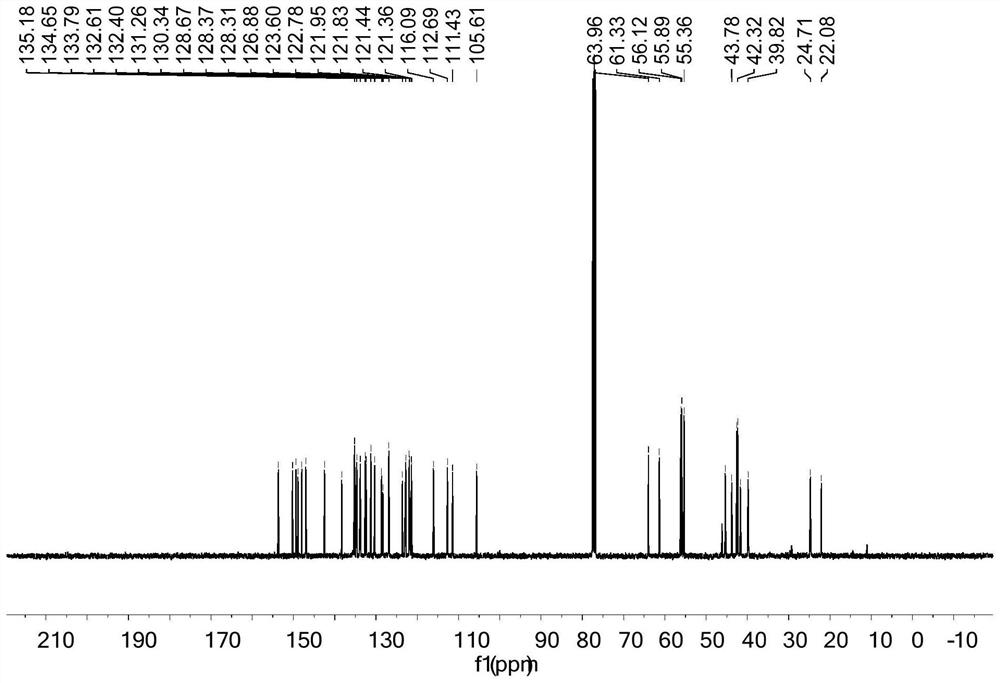
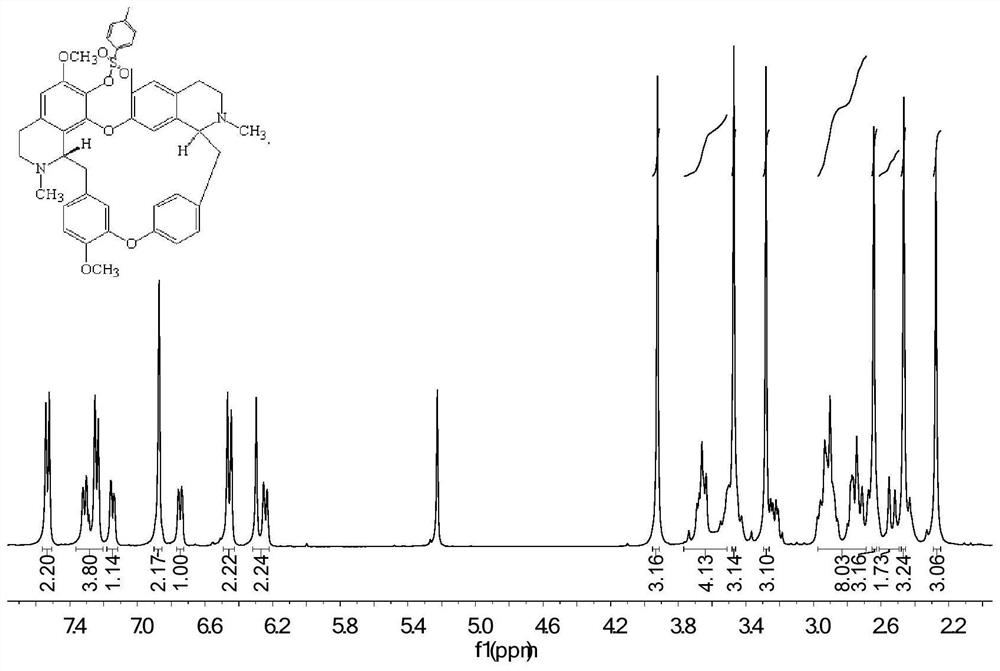
[0039]The preparation method of fangchinoline base derivatives with anticancer activity: 100.0 mg fangchinoline base dTet (about 0.18 mmol) was dissolved in 3 mL of dichloromethane, placed in a two-necked flask filled with argon, controlled Warm to 0°C. Under magnetic stirring, 66.5 mg (about 0.72 mmol) of triethylamine dissolved in 2 mL of dichloromethane was added. After 1 hour of reaction, 38.0 mg (about 0.20 mmol) of p-toluenesulfonyl chloride was added, and the reaction was tracked by TLC. After the reaction, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was dichloromethane:methanol (120:1, 100:1, 80:1), separated and purified to obtain the white powder product 119.5 mg (about 0.157 mmol), the yield is 87%, analyzed by NMR and HRMS, it is 7-O-(p-toluenesulfonyl) fangchinoline base, the NMR spectrum is as follows image 3 and 4 shown.
[0040] 1 H NMR (400 MHz, CDCl 3 ) δ 7.53 (d, J = 8.1 Hz, 2H), 7.36 – 7.20 (m,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com