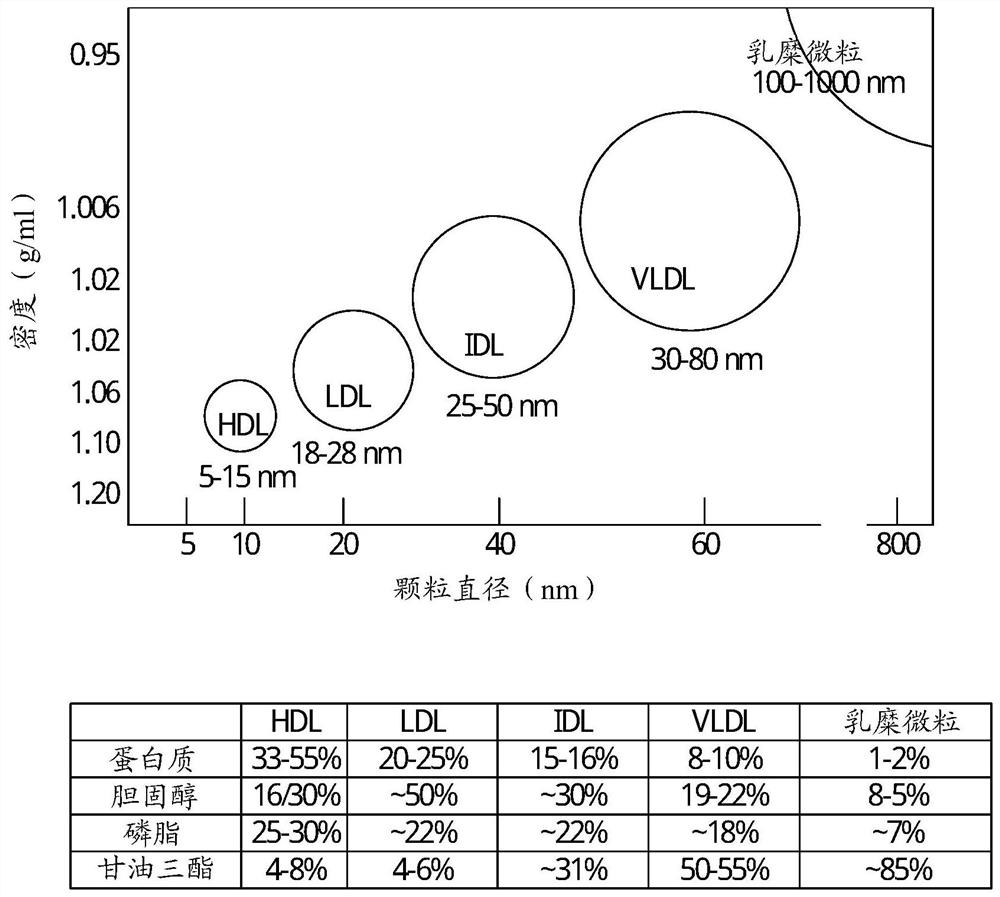
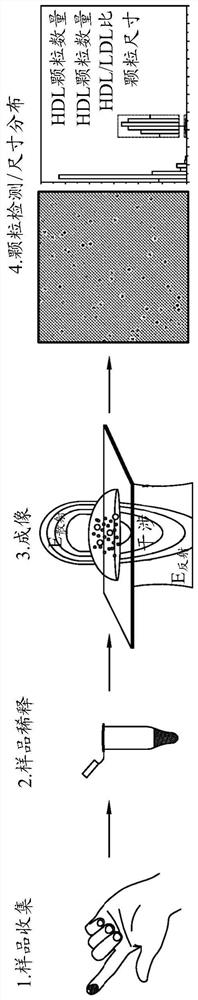
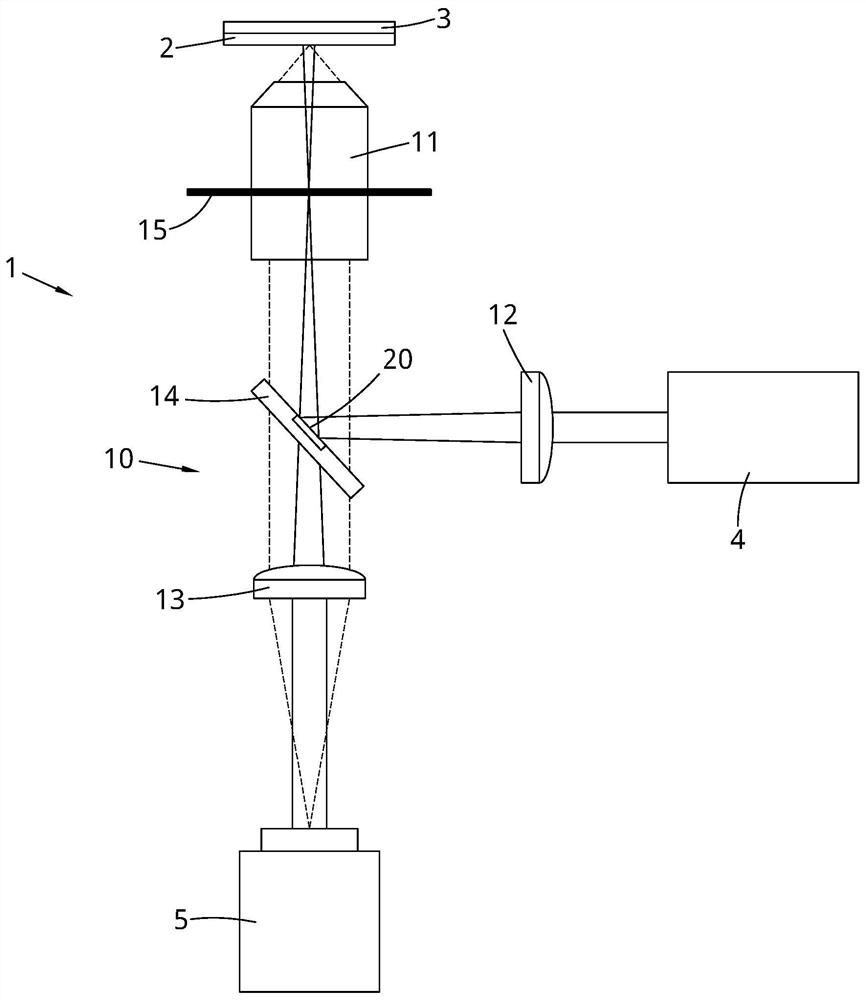
Method of determining lipoprotein concentration in solution using light scattering
A technology of light scattering and lipoprotein particles, which is applied to the measurement of scattering characteristics, analysis of suspensions and porous materials, and material analysis through optical means, and can solve problems such as expensive, incomplete information on lipoprotein particles, and limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Example 1 - Detection of purified lipoprotein particles by iSCAT
[0234] Materials / Methods
[0235] Substrate preparation and measurements
[0236] Borosilicate glass coverslips (No 1.5, 24 x 50 mm, VWR) were sequentially rinsed with MilliQ water, then with ethanol and again with MilliQ water. They were then dried under a stream of dry nitrogen. CultureWell silicone spacers (Grace Bio-Labs) were cut and placed on freshly cleaned coverslips to provide four separate 30-50 μl sample chambers on the same substrate.
[0237] Purified HDL and LDL lipoprotein samples were obtained from Lee Biosystems (LDL cat#360-10; HDL cat#361-10), see for example https: / / www.leebio.com / product / 984 / low-density-lipoprotein - described in ldl-human-serum-360-10. HDL and LDL were separated by ultracentrifugation. The samples were diluted 500,000-fold and 50,000-fold, respectively.
[0238] Data acquisition and analysis were then performed by iSCAT, while non-specific binding of lipopro...
Embodiment 2
[0241] Example 2 - Detection of Purified Lipoprotein Particles in Blood and Serum by iSCAT
[0242] The iSCAT method as described in Example 1 was performed on serum and blood samples from human individuals. To obtain serum, blood was allowed to clot in an upright position for at least 30 minutes and then centrifuged (30 minutes, 1500 xg). Transfer serum to plastic screw cap vials. Blood and serum samples were prepared as follows: 1 μL finger prick blood or serum samples were diluted 2000-fold in HEPES / KCl buffer containing 5 mM EDTA (to prevent clotting) (see below). 10 μl of the diluted sample was added to 40 μl of 25 mM HEPES buffer (pH 7.4, containing 100 mM KCl) to obtain a final 10,000-fold diluted blood.
[0243] Figure 5 Detection of HDL and LDL in plasma samples is shown. Imaged plasma samples simultaneously exhibited two signals corresponding to HDL and LDL. As expected, HDL particles appeared more frequently than LDL (see histogram below).
[0244] Figure...
Embodiment 3
[0245] Example 3 - Calibration to calculate HDL and LDL concentrations
[0246] To calculate the mass / concentration of HDL and LDL in the blood samples of Example 2, we constructed a calibration curve in which proteins of known mass / concentration were measured, giving a linear relationship between molecular weight and iSCAT contrast.
[0247] For concentration calibration, we used known concentrations of MSP1D1 DMPC lipid nanodiscs. Nanodiscs are synthetic model membrane systems composed of a lipid bilayer of phospholipids with a hydrophobic edge screened out by two amphipathic proteins. These proteins are called membrane scaffold proteins (MSPs) and are arranged in double bands. Nanodisc samples were diluted to the nM range and added to the buffer. The non-specific binding of nanodiscs to glass was recorded using iSCAT, as shown in Figure 7 shown. As the number of particles in solution decreases and the number of accessible binding sites decreases, the frequency of bin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com