Methods Used to Assess Risk in Clinical Trials
A clinical trial and risk technology, applied in the field of assessing the risk of clinical trials, can solve the problems that the risk evaluation parameters are not clearly disclosed, the risk evaluation model is not clearly disclosed, and cannot meet the needs of use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0098] The present invention will be described in detail below in conjunction with specific embodiments shown in the accompanying drawings. However, these embodiments do not limit the present invention, and any structural, method, or functional changes made by those skilled in the art according to these embodiments are included in the protection scope of the present invention.
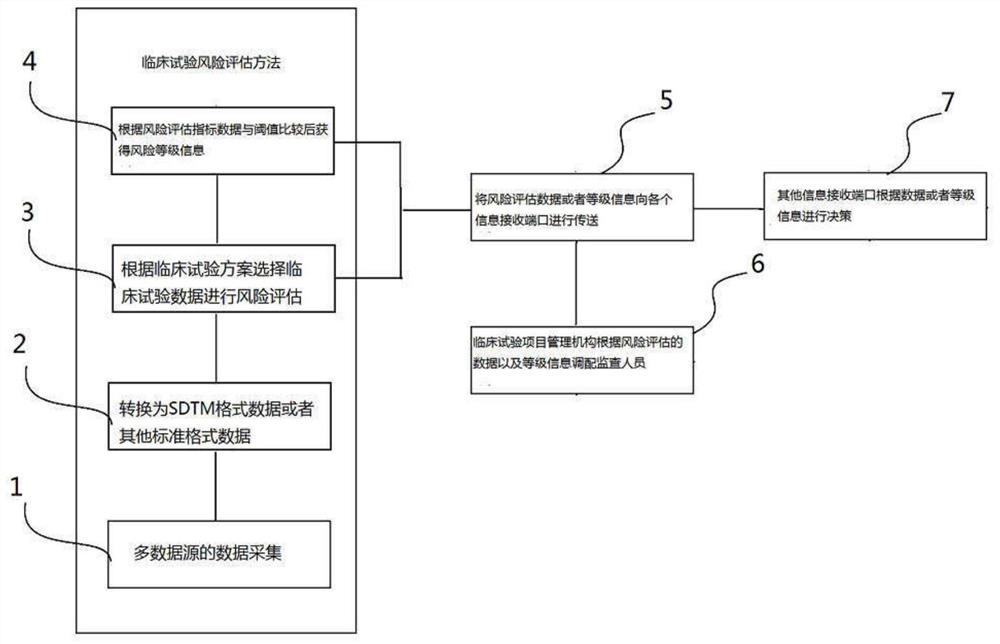
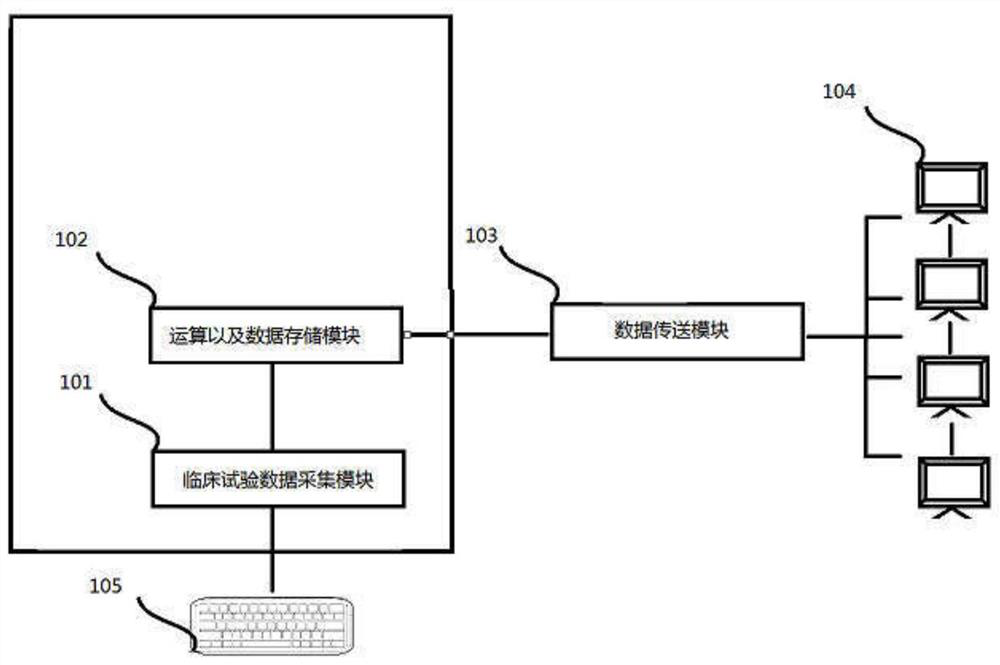
[0099] Please refer to the figure, a risk assessment method for clinical trials, the flow chart of the steps is as follows figure 1As mentioned above, it is applied to the system of clinical risk assessment, and the system is composed of figure 2 shown.
[0100] Specifically,
[0101] Multi-source test data collection step 1, multi-source test data collection step 1 is in figure 2 It is completed in the clinical test collection module 101, which is input through the data input device 105 or directly imported into the clinical test collection module 102 from various test systems through various dat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com