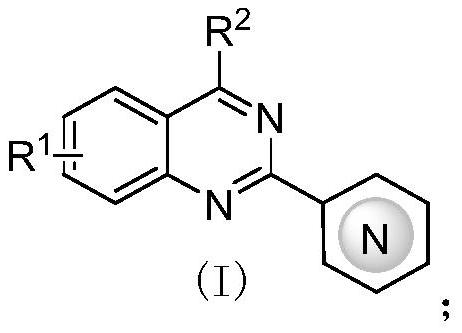
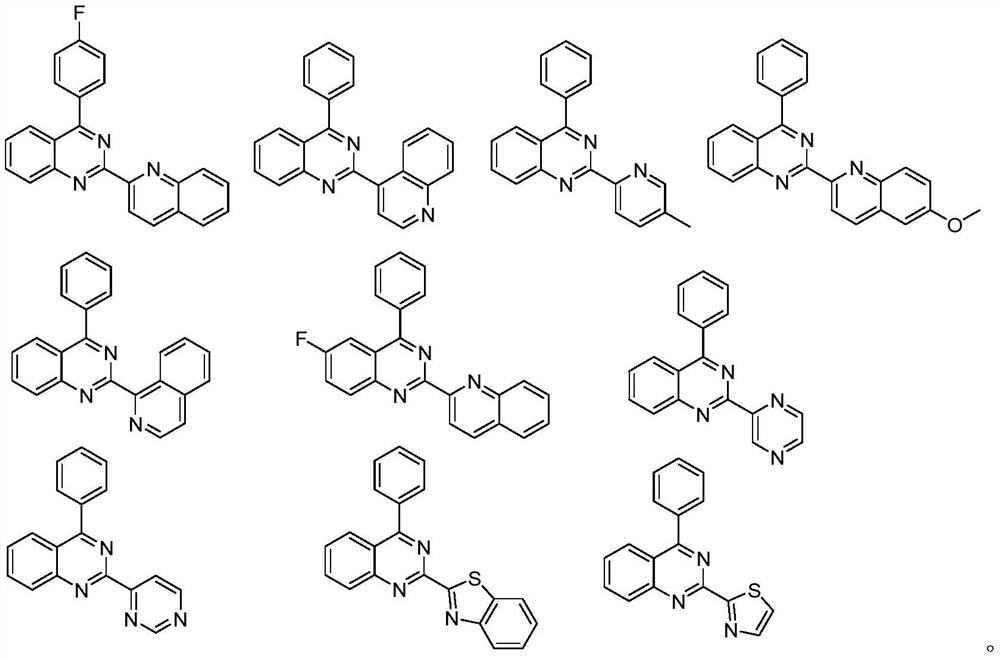
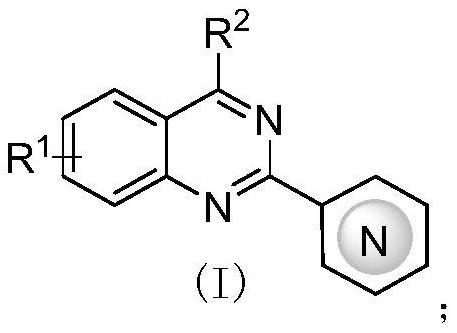
Application of 2-aza-arene-substituted quinazoline compound to preparation of antitumor drug
An anti-tumor drug, quinazoline technology, which is applied in the field of medicine, can solve problems such as the difficulty in the synthesis of quinazoline compounds, and achieve the effects of simple and efficient preparation methods, easy-to-obtain raw materials, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] 4-(4-fluorophenyl)-2-(quinolin-2-yl)-quinazoline: 74% yield, 1 H NMR (400MHz, CDCl 3 ) δ8.85(d, J=8.5Hz, 1H), 8.44(dd, J=18.6, 8.4Hz, 2H), 8.35(d, J=8.3Hz, 1H), 8.15(d, J=8.1Hz, 1H), 7.96(s, 3H), 7.88(d, J=7.7Hz, 1H), 7.77(t, J=7.4Hz, 1H), 7.66-7.58 (m, 2H), 7.31(t, J=8.3 Hz,2H). 13 C NMR (100MHz, CDCl 3 )δ167.6,163.9(d,J=248.9 Hz),159.0,155.1,151.9,148.2,136.8,133.7,133.3,132.3,132.2,130.7,129.9,129.5,128.4,128.1,1267.3,116.39,112 (d, J = 21.5 Hz).
Embodiment 2
[0046]
[0047] 4-Phenyl-2-(quinolin-4-yl)-quinazoline: 73% yield, 1 H NMR (400MHz, CDCl 3 )δ9.09 (d, J = 4.4Hz, 1H), 8.89 (d, J = 8.1Hz, 1H), 8.24 (t, J = 9.0Hz, 3H), 8.16 (d, J = 4.4Hz, 1H) , 8.01–7.95(m,1H),7.89(dd,J=6.6,2.9Hz,2H),7.79–7.74(m,1H),7.71–7.57(m,5H). 13 C NMR (100MHz, CDCl 3 )δ 168.8, 160.5, 151.5, 150.0, 149.1, 144.0, 137.0, 134.1, 130.2, 130.1, 129.8, 129.3, 128.7, 128.3, 127.2, 127.1, 126.3, 126.0, 122.8, 121.6.
Embodiment 3
[0049]
[0050] 4-Phenyl-2-(5-methylpyridin-2-yl)-quinazoline: 50% yield, 1 H NMR (400MHz, CDCl 3 )δ 8.74(s,1H),8.66(d,J=7.9Hz,1H),8.35(d,J=8.3Hz,1H),8.14(d,J=8.3Hz,1H),7.94 –7.86(m ,3H),7.68(d,J=7.6Hz,1H),7.58(s,4H),2.44(s,3H). 13 C NMR (101MHz, CDCl 3 )δ 168.7, 159.2, 152.7, 152.0, 150.7, 137.4, 137.4, 134.6, 133.7, 130.2, 129.9, 129.8, 128.5, 127.6, 126.9, 123.9, 122.1, 18.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com