Active targeting response type polypeptide drug as well as preparation method and application thereof
An active targeting and responsive technology, applied in the medical field, can solve the problem of low expression, achieve low toxicity and side effects, achieve long-term treatment, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
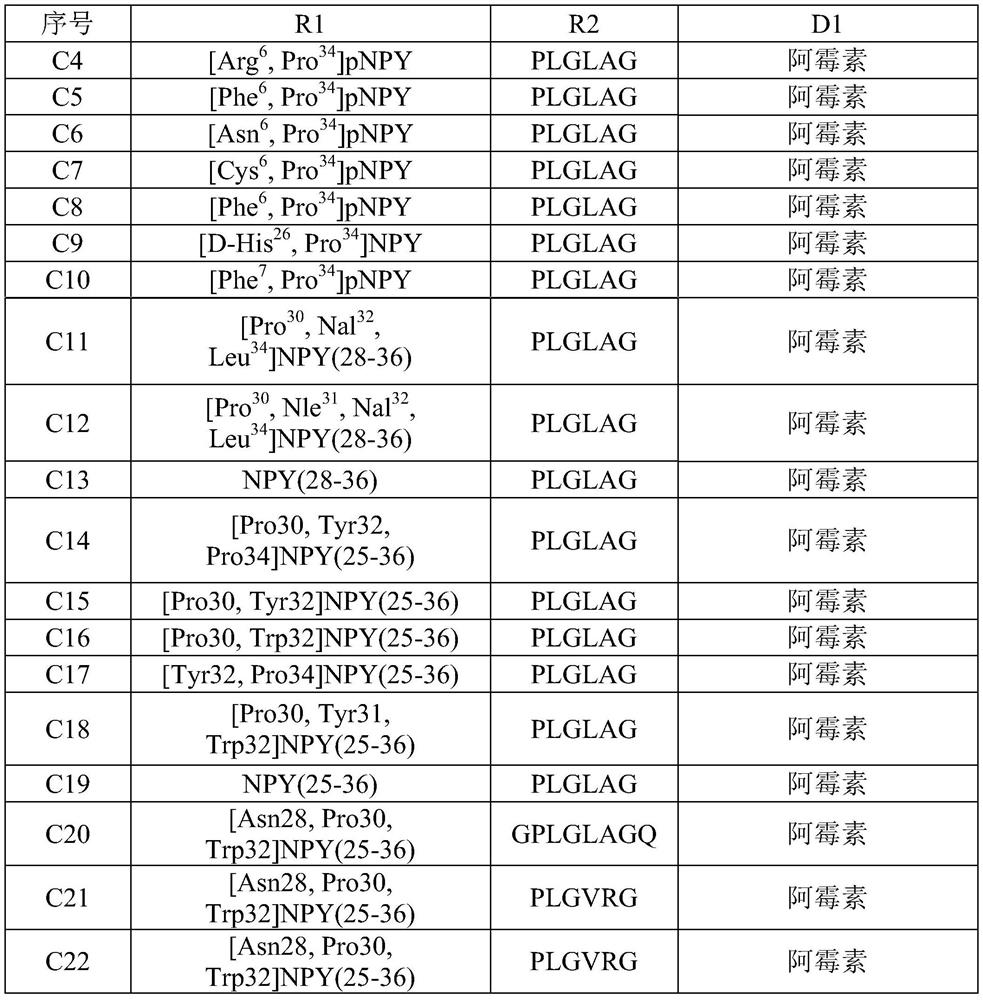
[0026] Synthesis of Example 1 Compound C1[Phe6, Pro34]pNPY-GPLGLLAGQ-Docetaxel
[0027] Compound C1 was synthesized according to the following steps:
[0028] PEG-1000 type polyurethane resin was selected as the substrate, and the peptide activated raw materials (Boc type) were placed one by one in the material box of the automatic peptide synthesizer according to the sequence, and the peptide synthesizer was started. After all the raw materials have been reacted, take a small amount of resin for cutting analysis, and after confirming that it is the desired polypeptide, put docetaxel with an activated carboxyl group in material tank 1 for reaction. After the reaction, a small amount of resin was taken for detection. After confirming the product, the synthesized resin was cleaved with 1% TFA to remove amino acid side chain protection, and then purified to a purity of 99% by preparative chromatography, and the purification process was self-monitored by preparative chromatograph...
Embodiment 2
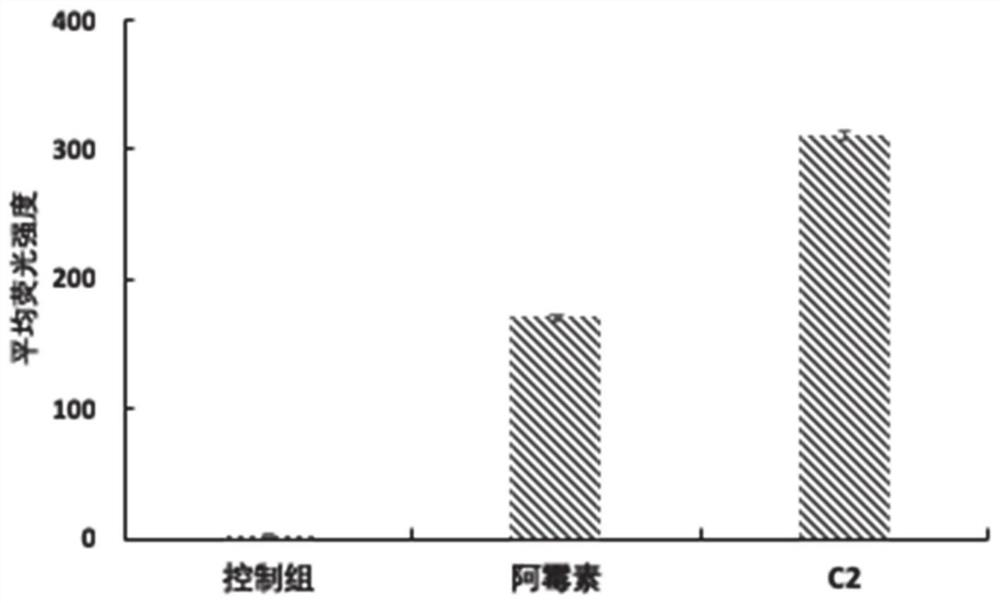
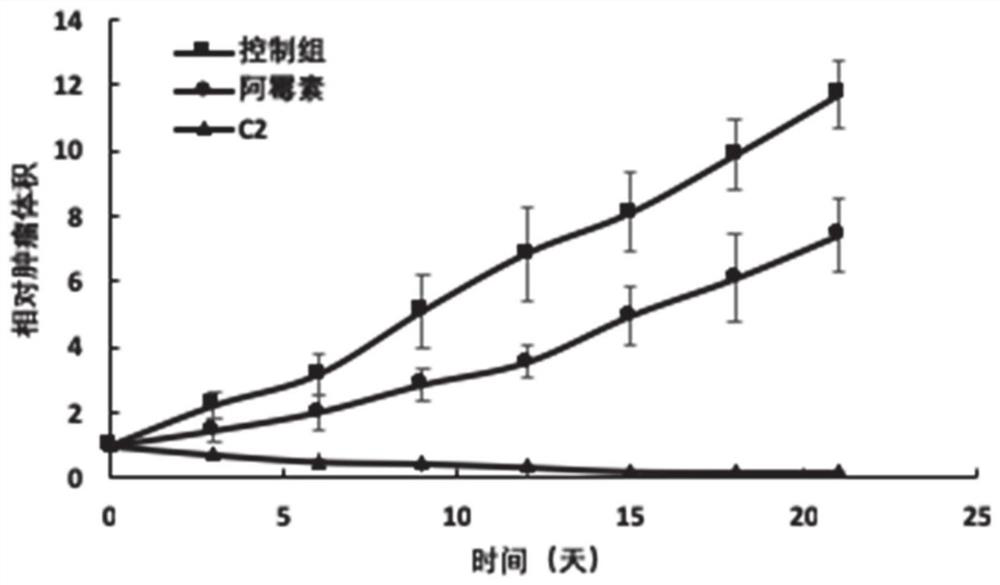
[0029] Synthesis of Example 2 Compound C2[Asn28, Pro30, Trp32]NPY(25-36)-PLGLAG-Adriamycin
[0030] Compound C2 was synthesized according to the following steps:
[0031] PEG-1000 type polyurethane resin was selected as the substrate, and the peptide activated raw materials (Boc type) were placed one by one in the material box of the automatic peptide synthesizer according to the sequence, and the peptide synthesizer was started. After all the raw materials have been reacted, take a small amount of resin for cutting analysis, and after confirming that it is the desired polypeptide, place doxorubicin with activated carboxyl groups in material tank 1 for reaction. After the reaction, a small amount of resin was taken for detection. After confirming the product, the synthesized resin was cleaved with 1% TFA to remove amino acid side chain protection, and then purified to a purity of 99% by preparative chromatography, and the purification process was self-monitored by preparative...
Embodiment 3
[0032] Synthesis of Example 3 Compound C3[Pro30, Nal32, Leu34]NPY(28-36)-PLGVRG-paclitaxel
[0033] Compound C3 was synthesized according to the following steps:
[0034] PEG-1000 type polyurethane resin was selected as the substrate, and the peptide activated raw materials (Boc type) were placed one by one in the material box of the automatic peptide synthesizer according to the sequence, and the peptide synthesizer was started. After all the raw materials have been reacted, take a small amount of resin for cutting analysis, and after confirming that it is the desired polypeptide, place paclitaxel with activated carboxyl groups in material tank 1 for reaction. After the reaction, a small amount of resin was taken for detection. After confirming the product, the synthesized resin was cleaved with 1% TFA to remove amino acid side chain protection, and then purified to a purity of 99% by preparative chromatography, and the purification process was self-monitored by preparative ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com