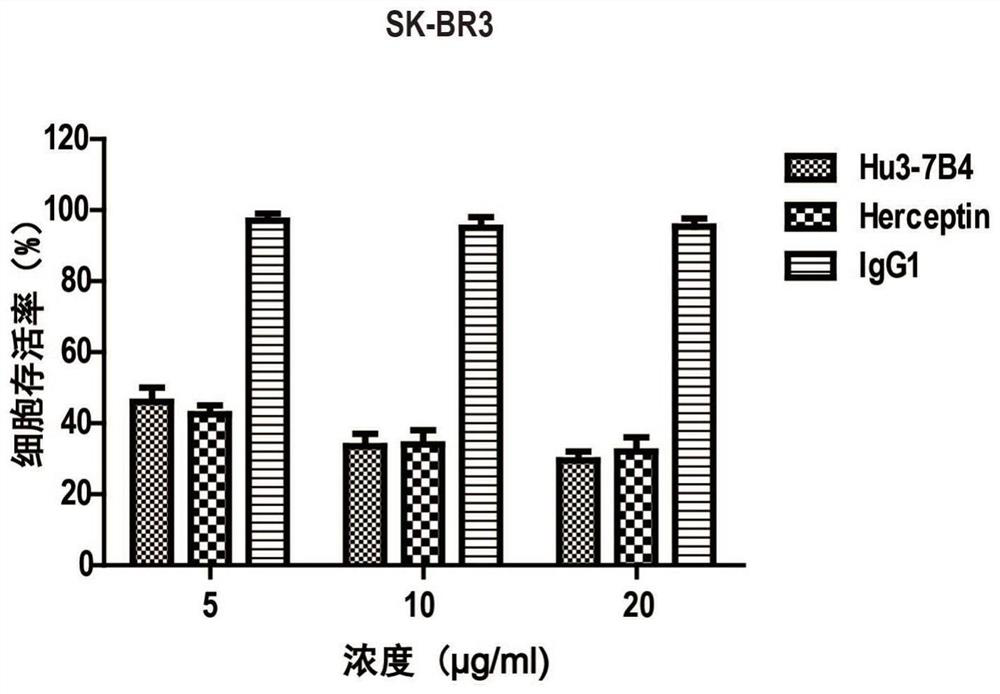
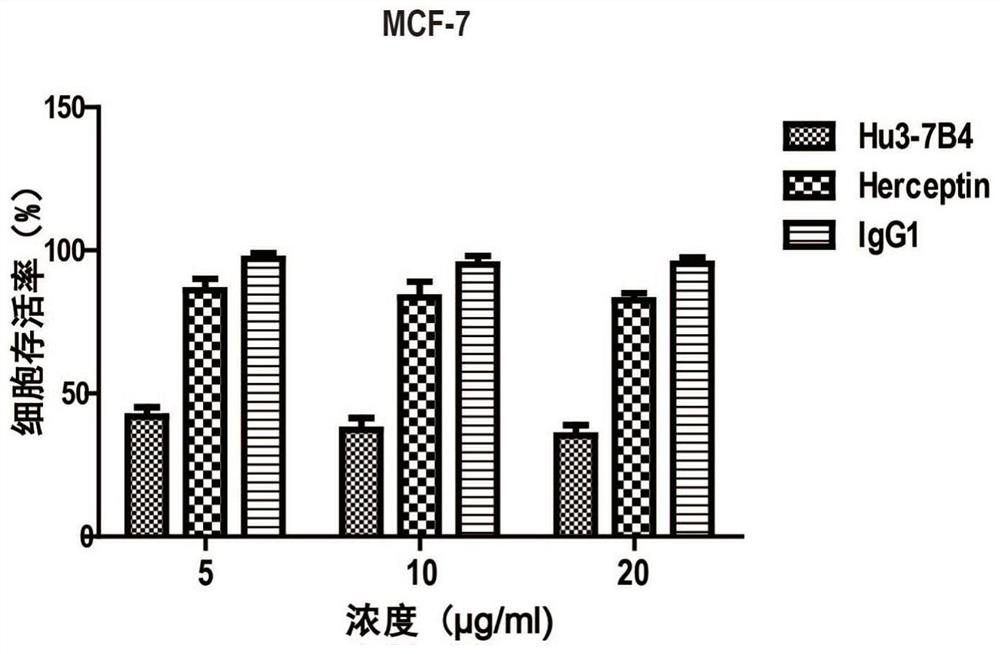
An anti-erbb2 antibody and its application in the treatment of breast cancer
An antibody and antigen technology, applied in the field of biomedicine, can solve the problem that the biological activity of antibodies needs to be further improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Preparation and purification of anti-ErbB2 antibody
[0029] Recombinant human ErbB2 extracellular domain (ErbB2-ECD) protein was used as immunogen, and Balb / c healthy female mice homologous to SP2 / 0 were selected as immunized animals. The immune antigen was dissolved in PBS, and mixed evenly with an equal volume of Freund's adjuvant to form a water-in-oil emulsion. Subcutaneous multi-point immunization was used for the first immunization, and the immunization dose was 100 μg / bird. 7-10 days after the initial immunization, use incomplete Freund's adjuvant to mix and emulsify with the same volume of immune antigen, and immunize mice with the same route and dosage. Afterwards, the immunization was boosted several times every 7-10 days, so that the antibody titer of the mouse serum reached more than 10,000. One week after the last booster immunization, blood was collected from eyeballs, and serum titers were determined by ELISA. If the mouse serum titer does ...
Embodiment 2
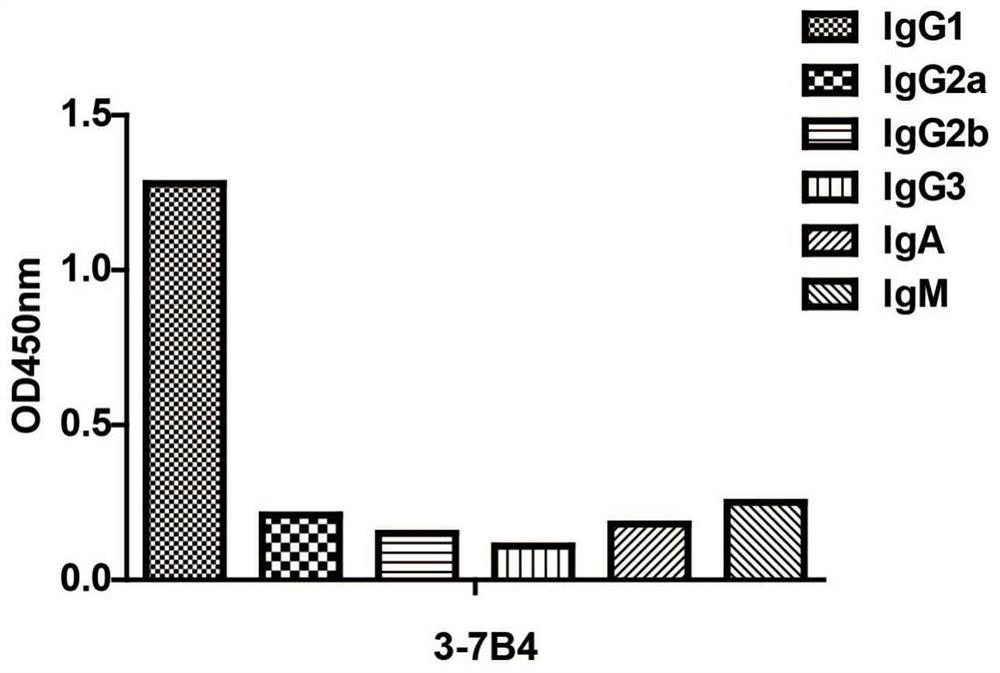
[0030] Example 2. Identification of mouse anti-ErbB2 antibody subtypes
[0031]The subtype of the anti-ErbB2 monoclonal antibody obtained in Example 1 was identified by ELISA (the kit was purchased from Proteintech). The ELISA plate provided in the kit has been pre-coated with specific antibodies against mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, kappa light chain, lambda light chain, and the anti-NSE purified in Example 2 Antibody samples E1-7E2 and E2-4F6 were added to the sample wells respectively, 50 μl per well, without incubation. Add 1X goat anti-mouse IgA+IgM+IgG-HRP into the sample wells, 50 μl per well, mix gently, and incubate for 1 h. Remove the liquid in the well and add 1XPBST to wash the well 3 times, and absorb excess water with absorbent paper. Add chromogenic solution, 100 μl per well, and develop color for 15 minutes at room temperature in the dark. Add 100 μl stop solution to stop the color reaction. Detect the OD value at 450nm by microplate reader, the...
Embodiment 3
[0032] Example 3. Antibody humanization
[0033] The positive hybridoma cells obtained in Example 1 were expanded and cultured, and an appropriate amount of cells were used for TRNzol-A + After lysis, each 1ml of TRNzol-A + Add 200 μl of chloroform, vortex for 15 seconds, and let stand for 3 minutes. Centrifuge at 13,000rpm at 4°C for 10 minutes. The cell solution is divided into three layers: the upper colorless aqueous phase, the middle layer and the lower yellow organic phase. Transfer the aqueous phase containing RNA to a centrifuge tube, and add an equal volume of isopropyl to the aqueous phase. alcohol, mix well, and let stand at room temperature for 25 minutes. Centrifuge at 13,000 rpm at 4°C for 10 minutes, discard the waste liquid to obtain the RNA pellet that sinks to the bottom. After washing the RNA pellet twice with 75% ethanol, the RNA was dissolved with DEPC water. Subsequently, the first strand of cDNA was synthesized using the TransScript One-Step gDNA Rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com