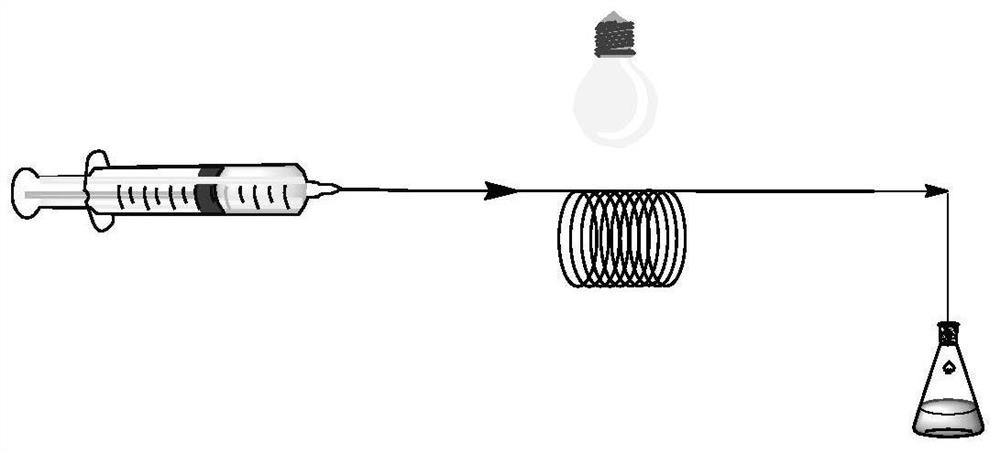
A method for the continuous photo-induced catalytic synthesis of pyrrolidone compounds
A technology of amine compounds and compounds, applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical chemical processes, etc., can solve problems such as low reaction efficiency and large metal residues, and achieve uniform illumination and metal-free Residue, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
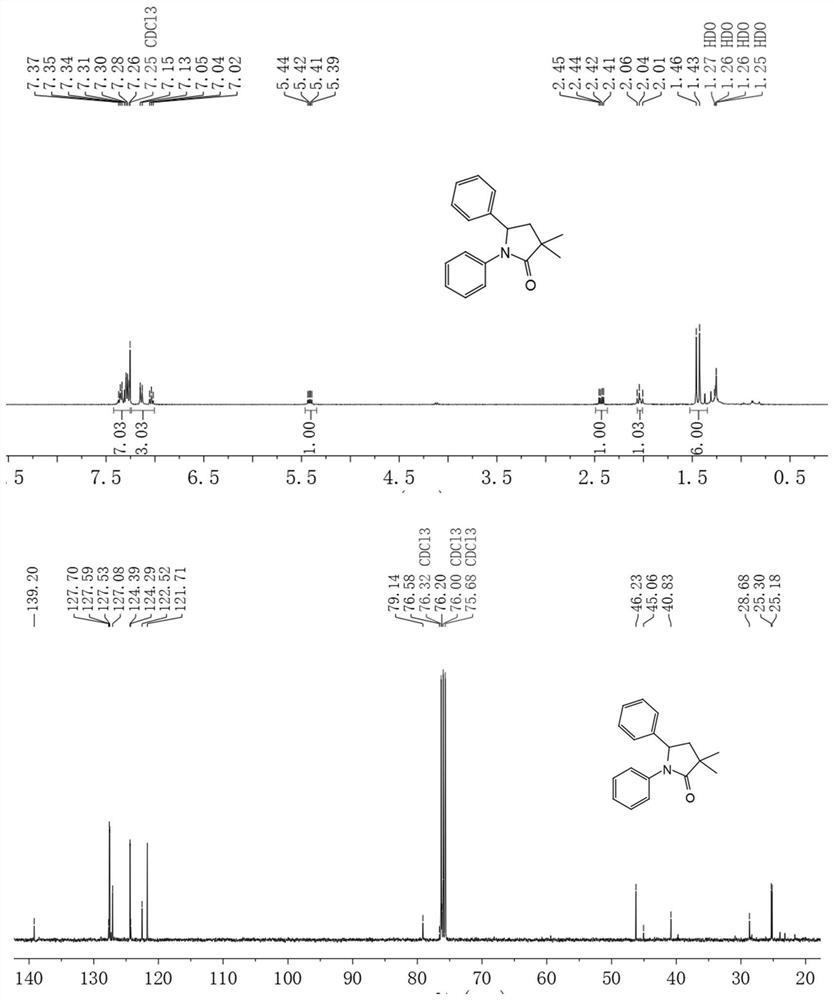
[0043]At room temperature, 5,10-bis(4-(trifluoromethyl)phenyl)-5,10-dihydrophenazine (23.5 mg, 50 μmol, 5% equivalent), 2-bromo-2-methylpropane Ethyl acetate (294.0 μL, 2 mmol, 2 equivalents), aniline (91.1 μL, 1 mmol, 1 equivalent) and styrene (116.8 μL, 1 mmol, 1 equivalent) were dissolved in the solvent DCE (4 mL), and stirred with a stirring bar, Exposure to simulated sunlight for 4 hours. The eluent of petroleum ether and ethyl acetate 10:1 (volume ratio) was used to separate, and the obtained product was vacuum-dried for 4 h. The conversion rate was 86%.
[0044] 1 H NMR (400MHz, Chloroform-d) δ7.42–7.25(m,7H),7.24–7.01(m,3H),5.42(dd,J=10.2,6.0Hz,1H),2.43(dd,J=12.7 ,6.0Hz,1H),2.04(s,1H),1.44(d,J=13.4Hz,6H). 13 CNMR (101MHz, Chloroform-d) δ139.20, 127.70, 127.59, 127.53, 127.32, 127.08, 124.39, 124.29, 122.52, 121.71, 121.67, 121.43, 79.14, 45.06, 40.83, 39.304, 25 18 h 19 NO[M+H + ]: 266.1539; measured value: 266.1508.
Embodiment 2
[0046] At room temperature, 5,10-bis(4-(trifluoromethyl)phenyl)-5,10-dihydrophenazine (23.5 mg, 50 μmol, 5% equivalent), 2-bromo-2-methylpropane Ethyl acetate (294.0 μL, 2 mmol, 2 equivalents), aniline (91.1 μL, 1 mmol, 1 equivalent) and styrene (116.8 μL, 1 mmol, 1 equivalent) were dissolved in the solvent DCE (4 mL) to obtain a homogeneous solution, and The above-mentioned homogeneous solution is pumped into the microchannel reactor, and the flow rate of the pump is controlled to be 0.1mL / min. In the microchannel reactor simulated sunlight irradiation, the retention time is 10min; the outflow liquid is collected with a sampling flask. The eluent of petroleum ether and ethyl acetate 10:1 (volume ratio) was used to separate, and the obtained product was vacuum-dried for 4 h. The conversion rate was 97%.
[0047] 1 H NMR (400MHz, Chloroform-d) δ7.42–7.25(m,7H),7.24–7.01(m,3H),5.42(dd,J=10.2,6.0Hz,1H),2.43(dd,J=12.7 ,6.0Hz,1H),2.04(s,1H),1.44(d,J=13.4Hz,6H). 13 CNMR (101MHz,...
Embodiment 3
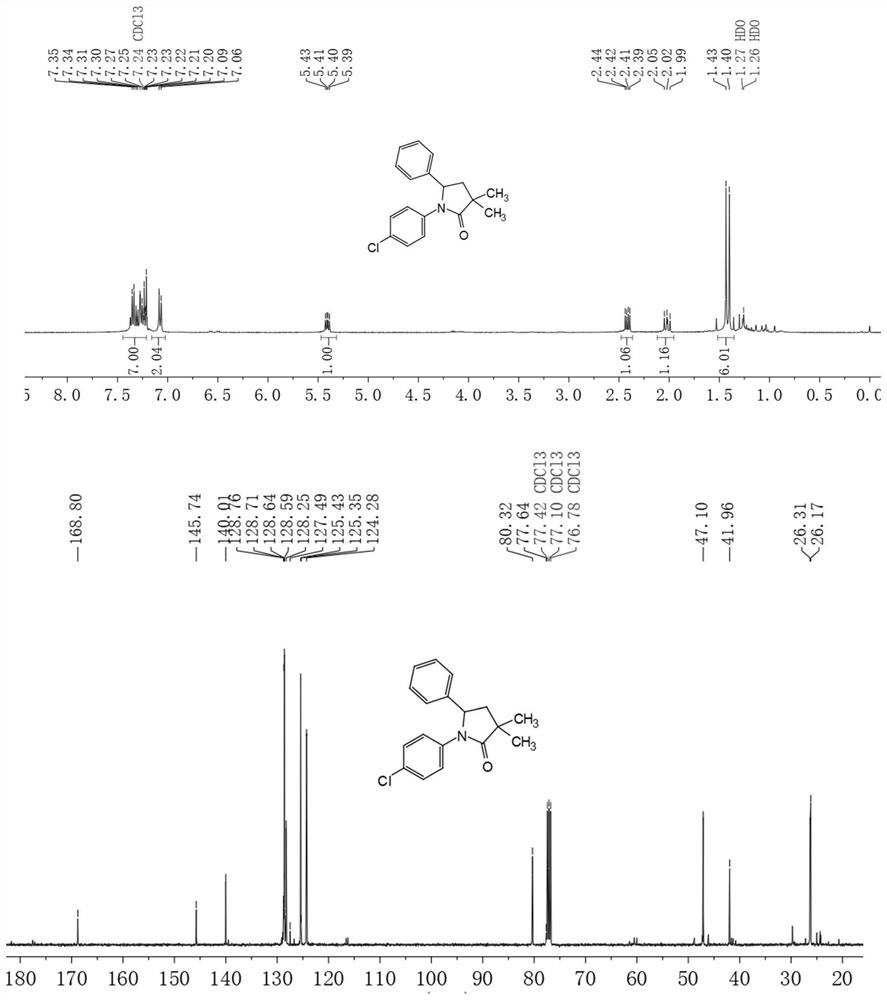
[0049] 5,10-Bis(4-(trifluoromethyl)phenyl)-5,10-dihydrophenazine (23.5 mg, 50 μmol, 5% equiv) was dissolved in solvent DCE (2 mL) at room temperature to give The first homogeneous solution; ethyl 2-bromo-2-methylpropanoate (294.0 μL, 2 mmol, 2 equiv), aniline (91.1 μL, 1 mmol, 1 equiv) and styrene (116.8 μL, 1 mmol, 1 equiv ) was dissolved in the solvent DCE (2mL) to obtain the second homogeneous solution; then the above-mentioned first second homogeneous solution was pumped into the microchannel reactor, and the control pump flow rate was 0.05mL / min. In the microchannel reactor, the retention time is 10 min; the effluent liquid is collected with a sampling flask. The eluent of petroleum ether and ethyl acetate 10:1 (volume ratio) was used to separate, and the obtained product was vacuum-dried for 4 h. The conversion rate was 96%. NMR see figure 2 .
[0050] 1 H NMR (400MHz, Chloroform-d) δ7.42–7.25(m,7H),7.24–7.01(m,3H),5.42(dd,J=10.2,6.0Hz,1H),2.43(dd,J=12.7 ,6.0Hz,1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com