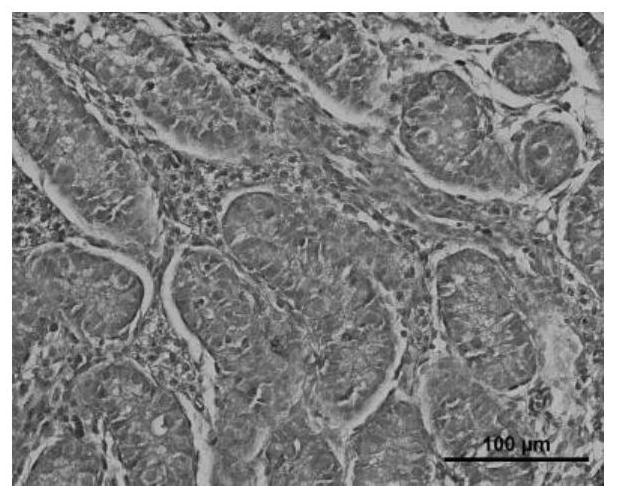
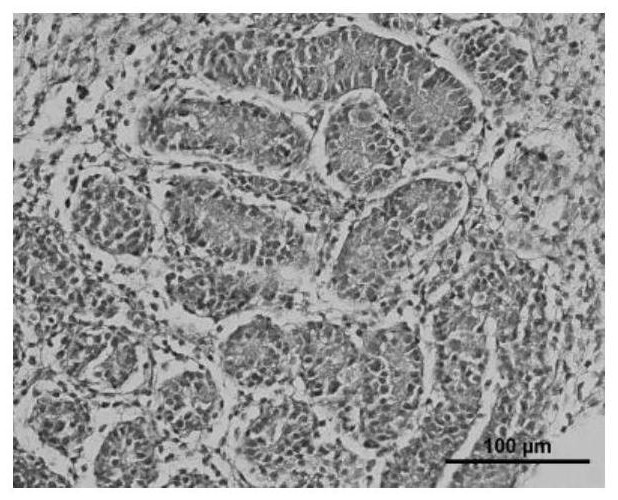
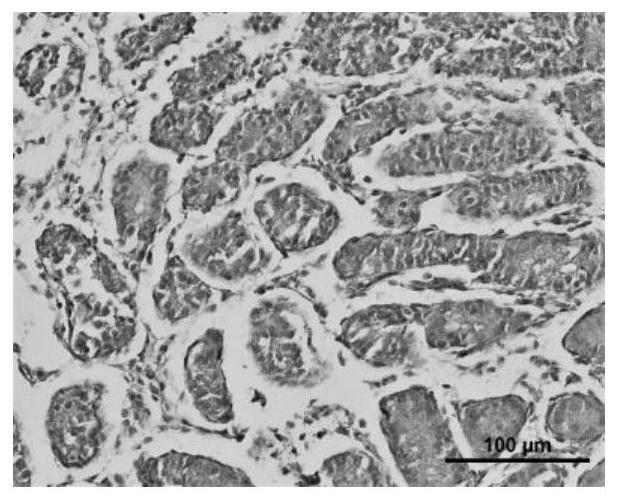
Application of cryoprotectant in organ and tissue cryopreservation
A cryopreservation solution and cryopreservation technology, applied in the field of biomedical materials, can solve the problems of affecting the survival rate of cryopreserved objects, safety function expression, cytotoxic side effects, and high toxicity of reagents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0113] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
[0114] The PVA used in the embodiment of the present invention has a syndiotacticity of 50%-55%, a molecular weight of 13-23kDa, and a degree of hydrolysis of 98%.
[0115] In the cryopreservation solution in the embodiment of the present invention...
Embodiment 1
[0117] Example 1: Cryopreservation of Oocytes and Embryos
[0118] 1. Preparation of cryopreservation solution: prepare cryopreservation solution according to the following formula
[0119] Cryopreservation solution A:
[0120] Each 100mL contains the following components:
[0121] substance content PVA (g) 2.0 Ethylene glycol (mL) 10 DMSO (mL) 10 Sucrose (mol L -1 )
0.5 Fetal bovine serum (mL) 20 DPBS (mL) margin
[0122] 2.0g of PVA was heated in a water bath at 80°C and dissolved in 25mL of DPBS with magnetic stirring. After the PVA was completely dissolved and cooled to room temperature, the pH was adjusted to 7.0, which was solution 1; 17g (0.05mol) of sucrose (sucrose was frozen The final concentration in the preservation solution is 0.5mol L -1 ) was ultrasonically dissolved in 25mL of DPBS. After the sucrose was completely dissolved, 10mL of ethylene glycol and 10mL of DMSO were added to form solution 2. After...
Embodiment 2
[0163] Example 2: Stem Cell Cryopreservation
[0164] Cryopreservation solution C1:
[0165] Each 100ml contains the following components:
[0166] substance Dosage PVA (g) 1.0 Ethylene glycol (mL) 10 Sucrose (mol L -1 )
[0167] Cryopreservation solution D:
[0168] Each 100ml contains the following components:
[0169] substance Dosage PVA (g) 2.0 Ethylene glycol (mL) 10 Sucrose (mol L -1 )
[0170] Cryopreservation solution F:
[0171] Each 100ml contains the following components:
[0172] substance Dosage Poly-L-arginine (g, degree of polymerization is 8) 4.0 PVA (g) 1.0 Ethylene glycol (ml) 10 Sucrose (mol L -1 )
[0173] The preparation method of the cryopreservation solution is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com