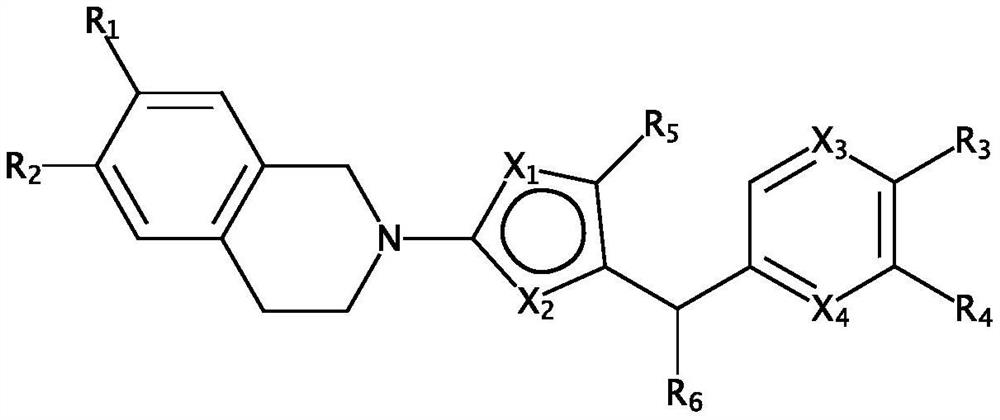
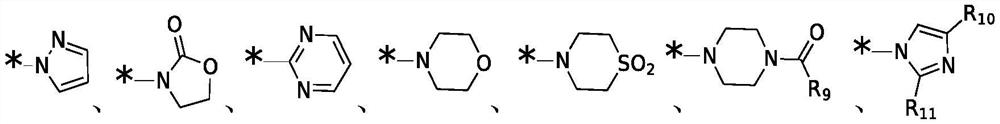
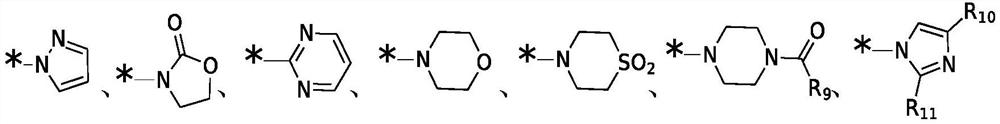
Broad spectrum antiviral compositions and methods
A technology of compositions and compounds, applied in the field of broad-spectrum antiviral compositions and methods, capable of solving problems such as reducing transplantation, infected organs, nephrotoxic side effects, and increased drug resistance rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0269] Preparation of intermediates
[0270] Preparation of Intermediate 1
[0271]
[0272] 1. 1 (Key Organics, 15g, 48.08mmol), 2,4-dimethyl-1H-imidazole (13.8g, 144.23mmol), (S,S)-N,N'-dimethyl-1, A mixture of 2-diaminocyclohexane (1.37g, 9.62mmol), t-BuOK (16.15g, 144.23mmol) and CuI (4.58g, 24.04mmol) in NMP (150mL) was heated at 160°C under N 2 Stir overnight. The mixture was cooled to RT and NaHCO was added 3 Saturated aqueous solution (50mL) and Boc 2 O (26.2 g, 120 mmol), and the resulting mixture was stirred overnight at RT. The mixture was concentrated and the residue was purified by chromatography on silica gel to provide material which was purified by prep-HPLC to afford 2 (6 g, 38% yield) as a pale yellow oil. MS (ESI): C 19 h 25 N 3 o 2 Theoretical molecular weight: 327.43, m / z experimental molecular weight: 327.9[M+H] + .
[0273] 2. To a solution of 2 (6 g, 18.35 mmol) in DCM (50 mL) was added TFA (50 mL). The resulting mixture was stirred overn...
Embodiment 1
[0436] 1. To a solution of Intermediate 7 (346 mg, 1.09 mmol) in DMSO (10 mL) was added Intermediate 9 (329 mg, 1.31 mmol) and K 2 CO 3 (300 mg, 2.18 mmol). The mixture was stirred overnight at 120 °C and cooled to RT. The mixture was treated with water and extracted with EA. The combined organic extracts were washed with water, brine, washed with anhydrous Na 2 SO 4 Dried, filtered and concentrated to provide a crude oil. The crude product was purified by silica gel chromatography to provide 90.36 mg of Example 1. 1 HNMR (CDCl 3 ,300MHz)δ:δ:1.3-1.4(d,6H),3.0-3.1(m,2H),3.6-3.8(m,2H),4.1(s,2H),4.2-4.3(s,2H), 4.8-4.9(s,2H),6.5(s,1H),7.0-7.1(s,1H),7.1-7.2(s,1H),7.4-7.5(s,1H),7.5-7.6(s,1H ), 7.7.6-7.7(d,1H), 7.7-7.8(s,1H), 7.8-7.9(s,1H), 7.9-8.0(s,1H). LC-MS: m / z=496.5(M+1) + .
Embodiment 2
[0438] 1. Intermediate 9 (100mg, 0.40mmol), Intermediate 3 (121mg, 0.44mmol), Pd 2 (dba) 3 (36.6mg, 0.04mmol), SPhos (16.4mg, 0.04mmol) and t-BuOK (123mg, 1.10mmol) in dioxane (2.00mL) was stirred at 95°C for 2h. The reaction was quenched with water and extracted with EtOAc. The combined organic extracts were washed with water and brine, washed with Na 2 SO 4 Dried and concentrated. The residue was purified by prep-HPLC to afford Example 2 (45.9 mg, 25% yield) as a yellow solid. 1 H NMR (400MHz, DMSO-d 6 )δ2.91(2H,t,J=5.6Hz),3.67(2H,t,J=5.6Hz),4.04(2H,s),4.08(3H,s),4.60(2H,s),6.53( 1H,t,J=2.0Hz),7.03(1H,s),7.25(1H,d,J=8.0Hz),7.36(2H,d,J=8.4Hz),7.63-7.73(3H,m), 7.77 (2H, d, J = 8.4 Hz), 8.46 (2 H, d, J = 2.0 Hz). MS theoretical value: 453.2; MS experimental value: 454.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com