Application of ciprofloxacin in preparation of human cytomegalovirus inhibitor
A technology of human cytomegalovirus and ciprofloxacin, which is applied in the directions of antiviral agents, medical preparations containing active ingredients, organic active ingredients, etc., to achieve the effect of preventing and/or treating diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
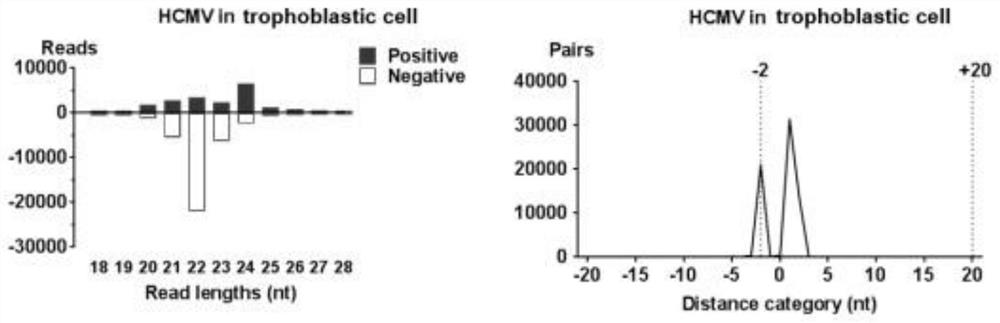
[0023] In this example, after HCMV infects trophoblast cells, it will stimulate the RNAi channel and produce siRNA. The specific process is as follows:
[0024] 1. Experimental materials
[0025] Human trophoblast cells, wild-type HCMV, trophoblast culture medium containing 10% Gibco serum and 2% double antibody, trophoblast medium containing 2% serum, total RNA extraction kit (Gibco).
[0026] 2. Experimental process
[0027] (1) Pave a 24-well plate with trophoblast cells;
[0028] (2) When it grows to 70%-80% confluence, replace the trophoblast culture medium containing 10% Gibco serum and 2% double antibody with the trophoblast medium containing 2% serum (per hole culture medium The amount added is 0.5ml), and 5μl10 was added to each well 7 PFU / ml wild-type HCMV;
[0029] (3) After 48 hours of virus infection, collect samples and extract RNA with a total RNA extraction kit;
[0030] (4) Discard the supernatant, add 350 μl TRK lysate to the well, and put it on the shak...
Embodiment 2
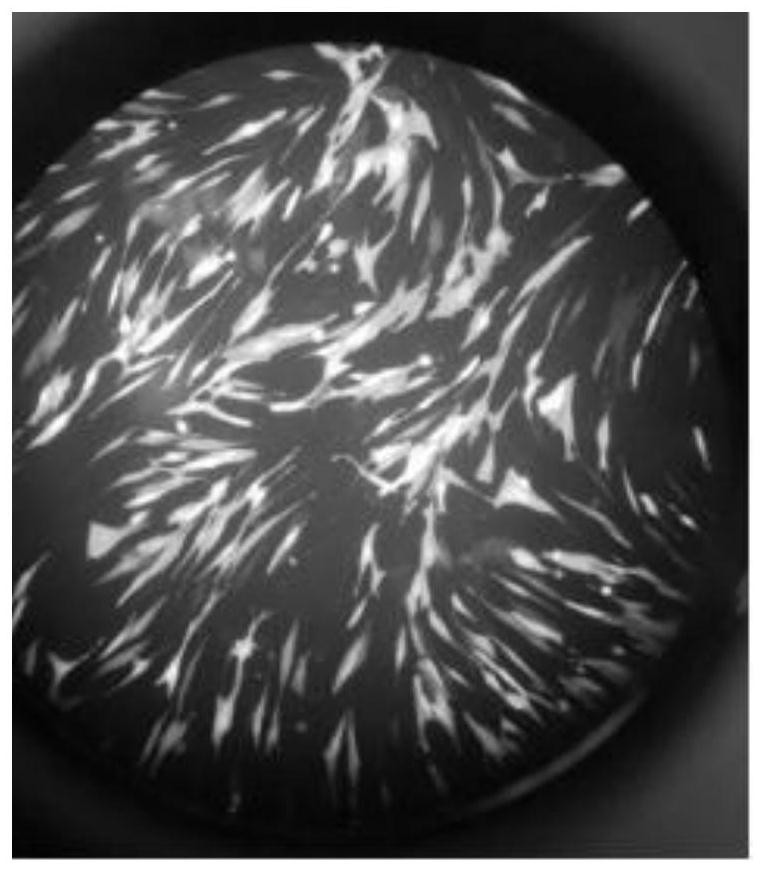
[0042] In this example, a HFF cell model infected with HCMV labeled with green fluorescent protein was established for subsequent research on the inhibitory effect of ciprofloxacin on HCMV. The specific process is as follows:
[0043] 1. Experimental materials
[0044] Human foreskin fibroblasts (HFF), GFP-HCMV labeled with green fluorescent protein, DMEM medium containing 10% Gibco serum and 2% double antibody, DMEM medium containing 2% serum, fluorescence microscope.
[0045] 2. Experimental process
[0046] (1) Utilize the reverse genetics system to construct the GFP-HCMV virus whose genome can express green fluorescent protein;
[0047] (2) Pave a 24-well plate with HFF cells;
[0048] (3) When it grows to 70%-80% confluence, replace the DMEM medium containing 10% Gibco serum and 2% double antibody with the DMEM medium containing 2% serum (the amount of culture medium in each well is 0.5ml), add 5μl 10 per well 6 PFU / ml GFP-HCMV;
[0049] (4) After virus infection 4 d...
Embodiment 3
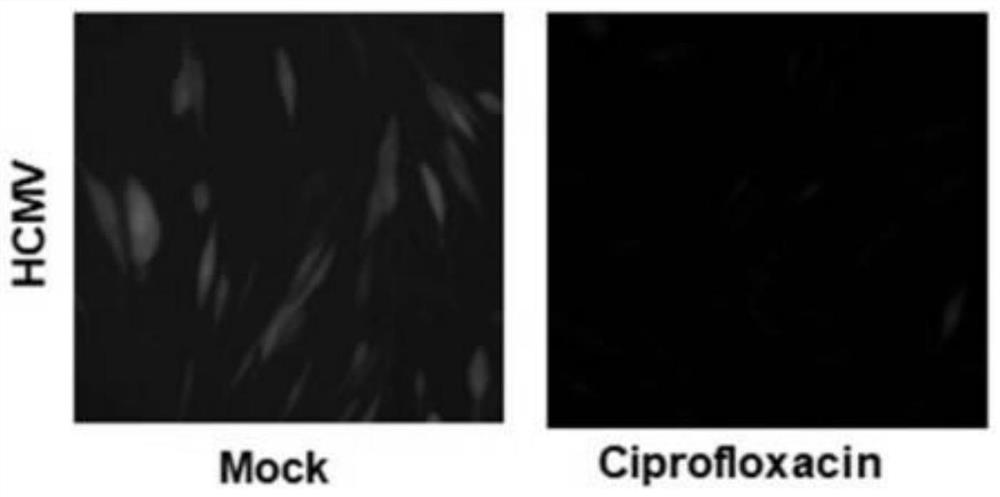
[0052] The present embodiment explores the inhibitory effect of ciprofloxacin (Cipro) on the viral level of HCMV, and the specific process is as follows:
[0053] 1. Experimental materials
[0054] Human foreskin fibroblasts (HFF), GFP-HCMV labeled with green fluorescent protein, DMEM medium containing 10% Gibco serum and 2% double antibody, DMEM medium containing 2% serum, PBS, fluorescence microscope.
[0055] 2. Experimental process
[0056] (1) spread HFF cells to 24-well plate;
[0057] (2) When it grows to 70%-80% confluence, replace the DMEM medium containing 10% Gibco serum and 2% double antibody with the DMEM medium containing 2% serum (the amount of culture medium in each well is 0.5ml), add 5μl 10 per well 7 PFU / ml GFP-HCMV;
[0058] (3) After virus infection 4h, abandon culture medium, wash twice with PBS, add the DMEM culture medium (the addition amount of every well culture medium is 0.5ml) freshly containing 2% serum, simultaneously in experimental group (be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com