Novel crystal form of esmolol hydrochloride and preparation method thereof
A technology of esmolol hydrochloride and crystal form, which is applied in the field of new crystal form and preparation of esmolol hydrochloride, can solve the problems affecting the efficacy of the final product, and achieve stable crystal form properties, strong operability, and dissolution good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 100ml reaction bottle, add 10g of esmolol hydrochloride, add 10ml of methanol, stir to dissolve, after the solid is completely dissolved, add 50ml of ethyl acetate at 15-25°C, keep at 15-25°C, stir and crystallize for 1-2 hours, Then the temperature was lowered to 0-10°C and stirred for 3-4h, the solid was filtered out, washed with a small amount of ethyl acetate, and vacuum-dried at 40°C for 4-6h to obtain 8.5g of off-white powder with a yield of 85%.
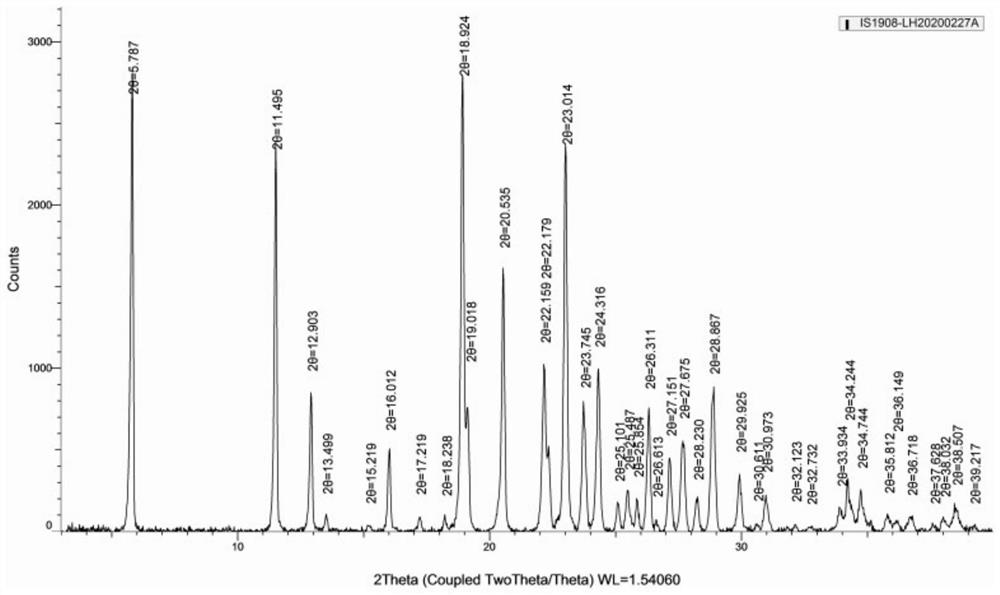
[0029] At ambient temperature and humidity, using Cu-Ka radiation, expressed in 2θ angles X-ray powder diffraction has characteristic peaks at the following positions: 2θ = 5.787°, 11.495°, 12.903°, 13.499°, 15.219°, 16.012°, 17.219 °, 18.238°, 18.924°, 20.535°, 22.159°, 23.014°, 23.745°, 24.316°, 26.311°, 27.675°, 28.887°, 29.93°, 30.973° have characteristic peaks, such as figure 1 shown.
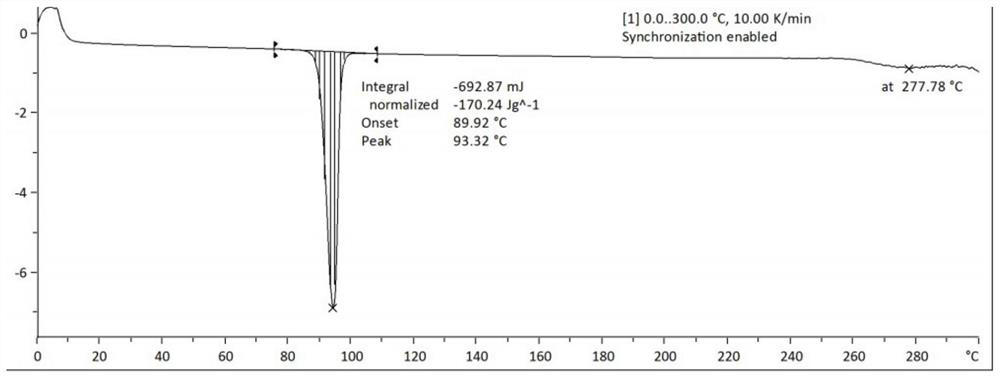
[0030] According to thermogravimetric analysis, the decomposition temperature of the crystal form A of esmolol hydrochlori...
Embodiment 2
[0035] In a 250ml reaction bottle, add 15g of esmolol hydrochloride, add 15ml of methanol, and stir to dissolve. After all the solids are dissolved, keep warm at 15-25°C and add 75ml of isopropyl acetate, keep warm at 15-25°C, stir and crystallize for 1-2 hour, then lower the temperature to 0-10°C and stir for 3-4h, filter out the solid, wash with a small amount of ethyl acetate, and vacuum-dry at 40°C for 4-6h to obtain 13.05g of off-white powder with a yield of 87%.
[0036] At ambient temperature and humidity, using Cu-Ka radiation, expressed in 2θ angles X-ray powder diffraction has characteristic peaks at the following positions: 2θ = 5.791°, 11.505°, 12.993°, 13.511°, 15.234°, 16.117°, 17.385 °, 18.369°, 19.018°, 20.662°, 22.287°, 23.198°, 23.902°, 24.501°, 26.502°, 27.799°, 28.968°, 30.911°, 32.545° have characteristic peaks.
[0037]According to thermogravimetric analysis, the decomposition temperature of the crystal form A of esmolol hydrochloride was 93.32° C., and t...
Embodiment 3
[0040] In a 250ml reaction bottle, add 15g of esmolol hydrochloride, add 30ml of isopropanol, stir to dissolve, after all the solids are dissolved, add 60ml of ethyl acetate at 25°C, keep at 15-25°C, stir and crystallize for 1-2 hours , then cooled to 0-10°C and stirred for 3-4h, filtered the solid, washed with a small amount of ethyl acetate, and dried in vacuum at 40°C for 4-6h to obtain 13.5g of off-white powder with a yield of 90%.
[0041] At ambient temperature and humidity, using Cu-Ka radiation, X-ray powder diffraction has characteristic peaks at the following positions in terms of 2θ angles: 2θ = 5.622°, 11.301°, 12.756°, 13.321°, 15.088°, 15.988°, 17.087 °, 18.099°, 18.796°, 20.388°, 22.004°, 22.886°, 23.596°, 24.156°, 26.159°, 27.495°, 28.706°, 29.759°, 30.795° have characteristic peaks.
[0042] According to thermogravimetric analysis, the decomposition temperature of the esmolol hydrochloride A crystal form A was 93.32 °C, and there was no weight loss before the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com