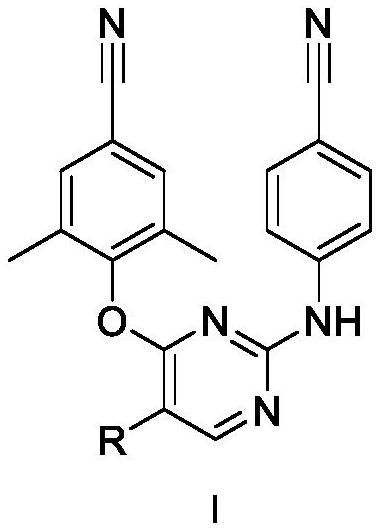
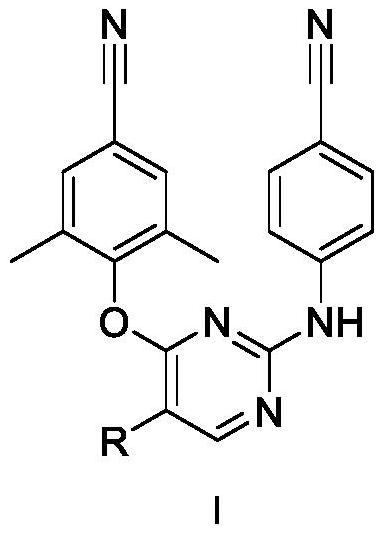
5-site aromatic ring substituted diarylpyrimidine derivative as well as preparation method and application thereof
A technology of derivatives and substituents, applied in the fields of diarylpyrimidine derivatives and their preparation, derivatives and their preparation, can solve problems such as side effects and poor oral availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
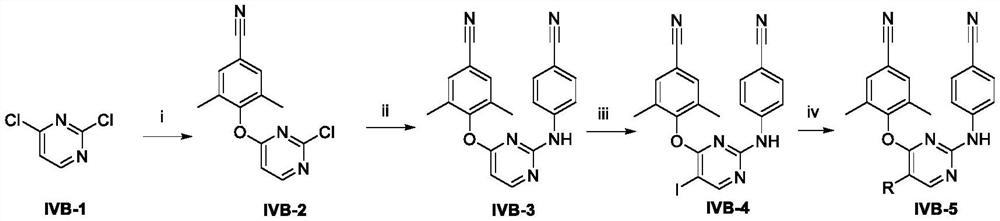
[0052] Example 1: Preparation of 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (IVB-2)
[0053] Weigh 2,4-dichloropyrimidine (IVB-1, 0.2g, 1.34mmol) and 4-hydroxy-3,5-dimethylbenzonitrile (0.16g, 1.07mmol) and dissolve in 20mL N,N-dimethyl In base formamide, add K 2 CO 3 (0.22g, 1.61mmol) and stirred overnight at room temperature. After the reaction, the above reaction solution was added dropwise to 150mL of water, a large amount of white solid was precipitated, filtered and dried in vacuum to obtain the intermediate IVB-2 with a yield of 86% and a melting point of 195-197°C.
Embodiment 2
[0054] Example 2: Preparation of 4-((2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (IVB-3)
[0055] Weigh palladium acetate (0.11g, 0.5mmol) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.29g, 0.5mmol) into 15mL 1,4-dioxane , stirred at room temperature for 15 min. Then the above intermediate IVB-2 (3.12g, 10mmol) and cesium carbonate (4.89g, 15mmol) were added and stirred for another 10min. Finally, p-aminobenzonitrile (1.18g, 10mmol) was added, heated to 80°C for 6h under nitrogen protection, cooled and filtered, then concentrated, dissolved ethyl acetate (80mL), water (20mL×3), saturated saline (20mL) Washing in sequence, drying with anhydrous sodium sulfate, and silica gel column chromatography after filtration to obtain white solid IVB-3 with a yield of 50% and a melting point of 275-277°C.
Embodiment 3
[0056] Example 3: 4-((2-((4-cyanophenyl)amino)-5-iodopyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (IVB-4) preparation
[0057] Weigh the above intermediate IVB-3 (0.94g, 3.3mmol) in a round bottom flask, add acetonitrile 20mL, add trifluoroacetic acid (1mL, 13.2mmol) and N-iodosuccinimide (0.97 g, 4.3mmol), continue stirring at room temperature. After the reaction, a white solid precipitated out. Filter and dry to obtain crude IVB-4. Yield: 97%, melting point: 283-285°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com