Preparation method of imatinib mesylate
A technology of imatinib mesylate and methanesulfonic acid, which is applied in the field of preparation of imatinib mesylate, can solve the problems of long reaction time, harsh reaction conditions, complicated and complicated post-processing, etc., and achieves easy operation. , The effect of reducing production cost and process optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] All features disclosed in this specification, or steps in all methods or processes disclosed, may be combined in any manner, except for mutually exclusive features and / or steps.
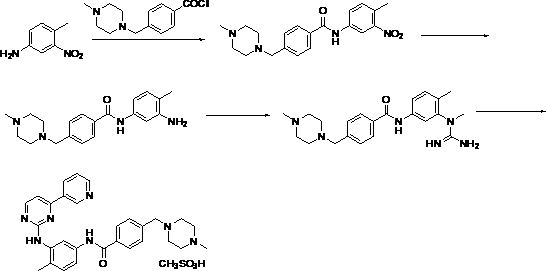
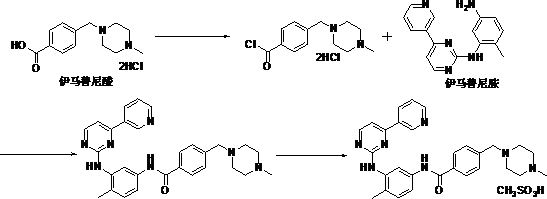
[0024] In this example, first use thionyl chloride to chlorinate imatinib acid [4-(4-methylpiperazin-1-ylmethyl)benzoic acid dihydrochloride] to generate imatinib mesylate Ni intermediate I; then condensed with imatinib amine [N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine] to obtain imatinib mesylate The Ni intermediate II; finally with methanesulfonic acid salt to obtain imatinib mesylate. Its chemical reaction formula is as follows:
[0025]
[0026] Wherein, the preparation operation process of imatinib mesylate intermediate I [4-(4-methylpiperazine methyl) benzoyl chloride dihydrochloride] is as follows:
[0027] (1) Add 25.0kg of imatinib acid, about 300kg of thionyl chloride, and 4-7kg of DMF into a 300L replacement reaction tank, first raise the temperature to 45-70°C a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com