Antibody binding tim-3 and use thereor
A TIM-3, antibody technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1 Phage panning, screening and affinity maturation
[0176] Phage library
[0177] Antibody single chain phage display libraries were generated by cloning repertoires of light chain variable regions (VL) and heavy chain variable regions (VH). The heavy and light chain repertoires were generated by PCR amplification of mainly peripherally collected human lymphocytes. The VL repertoire was mixed with the VH repertoire and PCR was performed with overlapping primers. The final form of the antibody is a single chain Fv (scFv) with a VH segment and a VL segment linked by a flexible linker peptide (GGGGSGGGGSGGGGS (SEQ ID NO: 51 )).
[0178] Phage library panning against human TIM3
[0179] Selection of phage particles displaying specific scFv fragments on Immuno 96 MicroWell TM plate (Nunc, Denmark). First, 50 μg / ml TIM3 recombinant protein (AcroBiosystems, catalog #TM3-H5229) in phosphate buffered saline (PBS) was coated on the plate overnight at 4°C. After b...
Embodiment 2
[0186] Embodiment 2 physicochemical analysis
[0187] Antibody TIM3-6 was tested in size exclusion chromatography. In particular, using 100mM sodium phosphate + 100mM Na 2 SO 4 , pH 7.0 as the running buffer, 20 μg of the sample was injected on a TSK G3000SWXL column. Run time is 30 minutes. All measurements were performed on Agilent1220HPLC. Data were analyzed using OpenLAB software.
[0188] In SEC, the main peak of antibody TIM3-6 is above 95%, which indicates that the purity and integrity of the purified antibody are high.
Embodiment 3
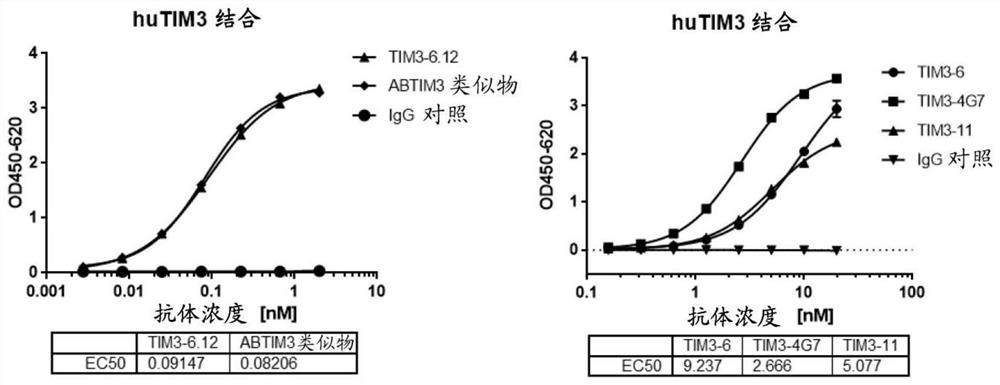
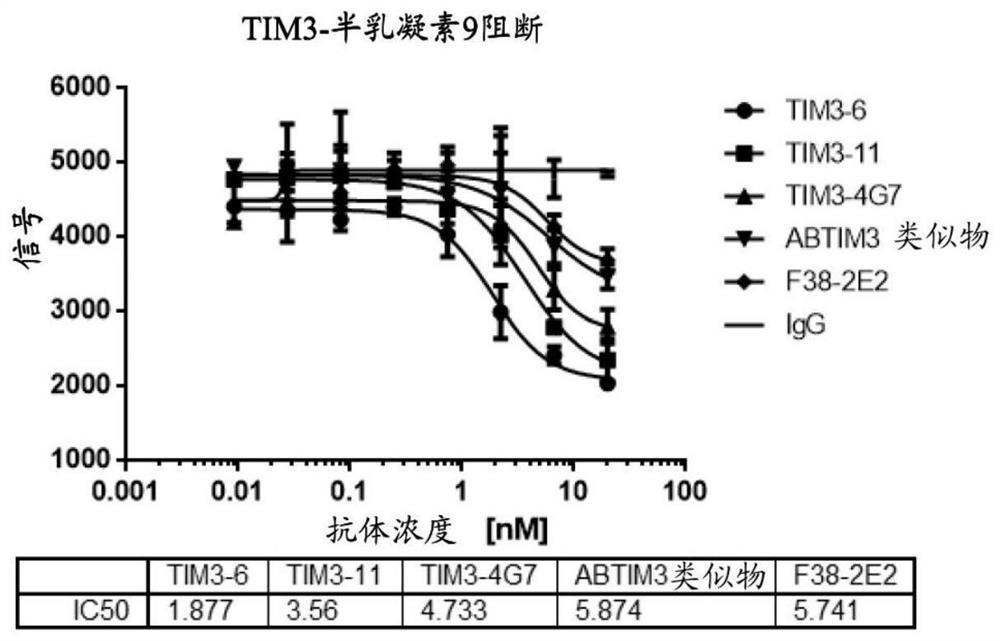
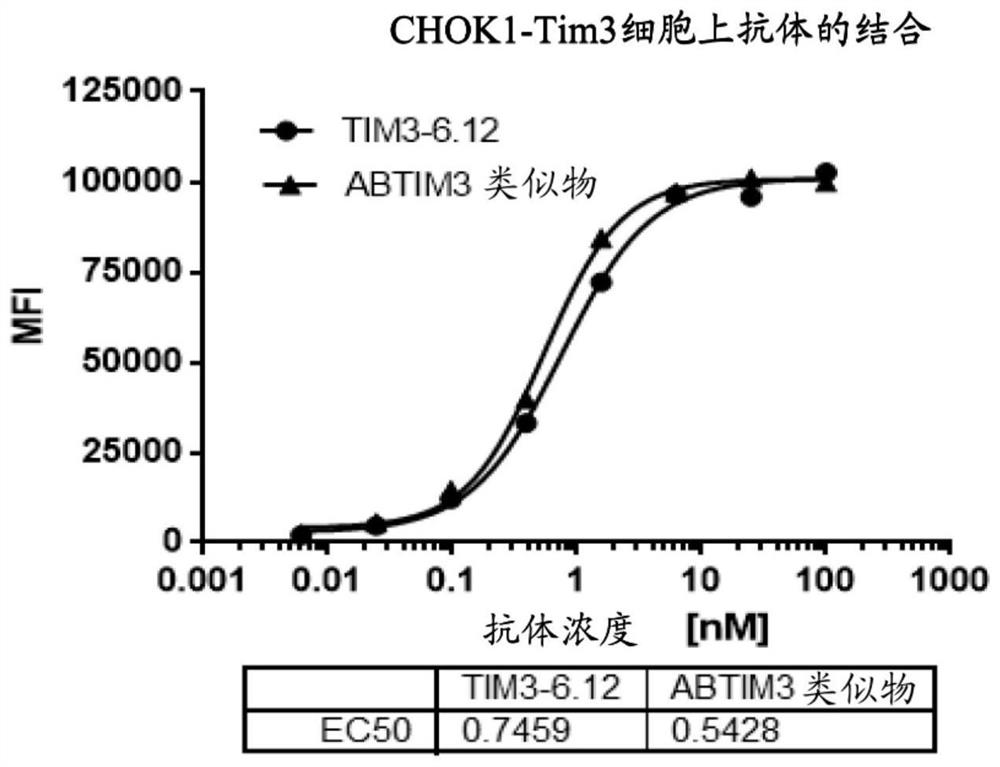
[0189] Example 3 Anti-TIM-3 antibody specifically binds to human TIM-3
[0190] ELISA assays were used to determine the relative binding activity of antibodies to recombinant human TIM-3.
[0191] Human TIM-3 protein (Acrobiosystems, catalog #TM3-H5229) in carbonate buffer (pH 9.6, 1.59 g sodium carbonate and 2.93 g sodium bicarbonate dissolved in 1 L of water) was immobilized by overnight incubation at 4 °C on a 96-well plate. Plates were then blocked with 1% BSA in PBS for one hour at 37°C. After blocking, the plates were washed three times with PBST (PBS containing 0.05% Tween20). Serially diluted anti-TIM-3 antibodies TIM3-6.12, TIM3-6, TIM3-11 and TIM3-4G7 in binding buffer (PBS containing 0.05% Tween20 and 0.5% BSA), human IgG control (which was prepared according to US20190016800A1 , having the amino acid sequence listed in SEQ ID NO:52) and ABTIM3 analogs (used as a reference antibody, prepared according to US2015 / 0218274A1, having the amino acid sequences of heav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com