iPSC residue detection method using ESRG gene as universal marker gene
A detection method and a general-purpose labeling technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of high cost, easy differentiation of iPSC, false positives, etc., and achieve low cost and improved High detection efficiency and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 ESRG gene is used as the validation test of universal marker gene
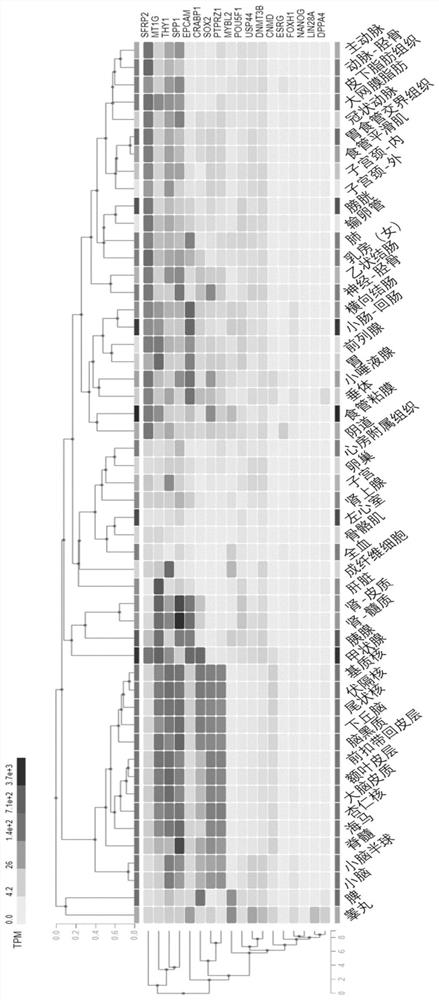
[0048] (1) Screen candidate genes: Search the expression of a series of genes in different human tissues through GTEx Portal official website, and screen candidate genes. The criteria for candidate genes are genes that are not expressed in human tissue cells, or are only expressed in a very small number of cells. figure 1 is the expression level (TPM) of each gene in different human tissues, according to figure 1 As shown, ESRG, lin28A and NANOG meet the above screening criteria, wherein ESRG and lin28A are only expressed in human reproductive organs; NANOG is not expressed in the tested tissues.
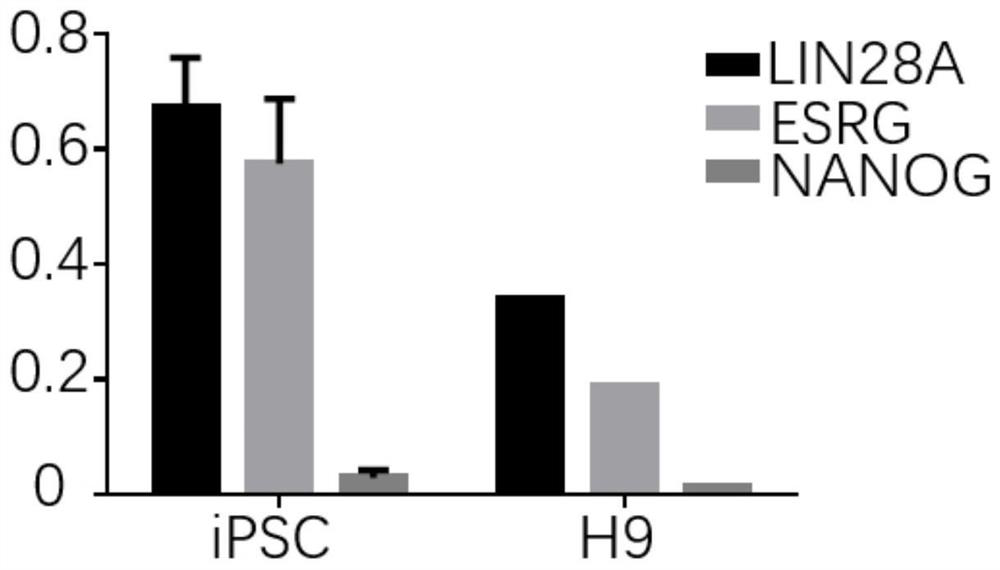
[0049] (2) Detect the expression levels of candidate genes in iPSCs and ESCs: the ESCs used are embryonic stem cells H9; first, the mRNA of iPSCs and embryonic stem cells H9 is extracted by the above-mentioned method of total mRNA extraction, and then the iPSCs and H9 are extracted by the above-men...
Embodiment 2
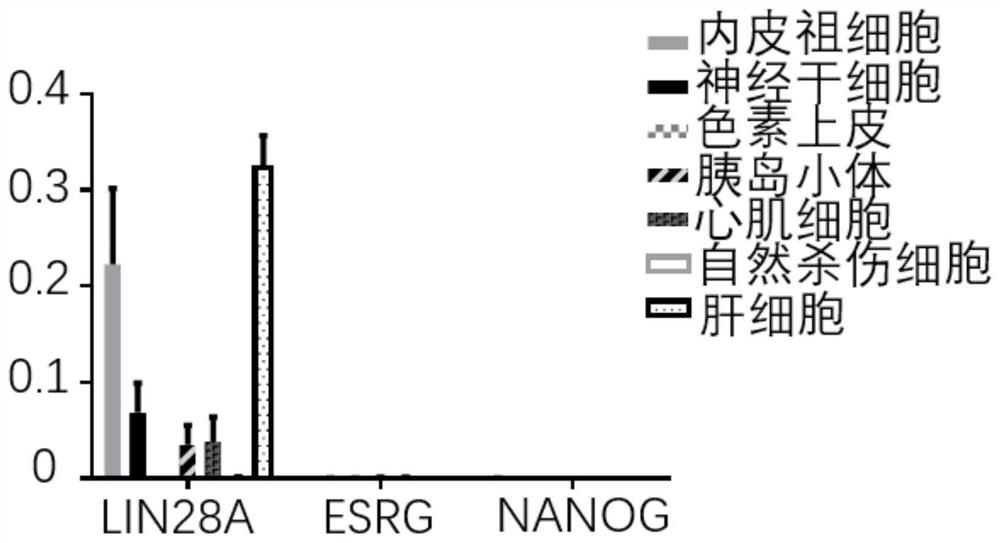
[0053] Example 2 Detection of iPSC residues in iPSC-derived iEPCs
[0054] (1) Design the structure of the ESRG marker gene: look up the cDNA sequence of ESRG by NCBI, and the nucleotide sequence list is as shown in sequence SEQ ID NO:1 (
[0055]
[0056]
[0057] ), the sequence length is 3153bp. Randomly select a sequence greater than 200bp as the target gene sequence constructed by the standard, and design primers according to the target gene sequence, wherein the length of the target gene sequence is greater than or equal to the length amplified by the primer; the ESRG cDNA target selected in this embodiment The sequence length is 338bp, and its nucleotide sequence is shown in the sequence SEQ ID NO: 2 ( ). In this embodiment, the primers are the above-mentioned ESRG upstream primer (ESRG-F) and ESRG downstream primer (ESRG-R).
[0058] In this embodiment, the standard product is plasmid DNA containing the ESRG target gene sequence; the advantage of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com