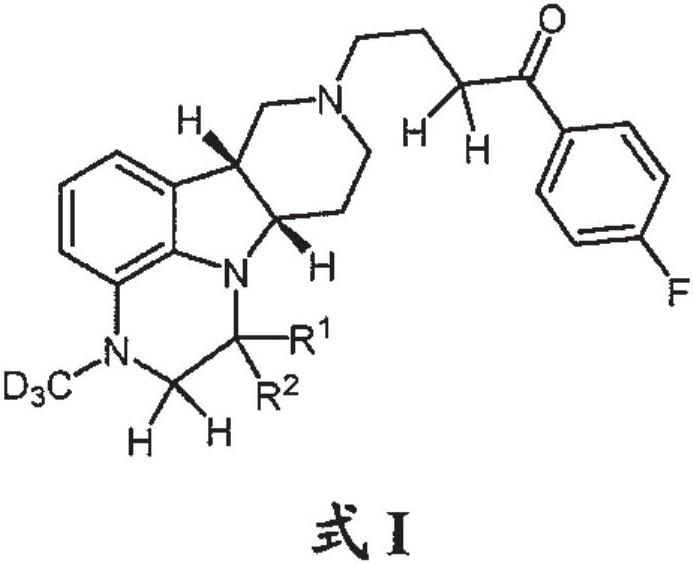
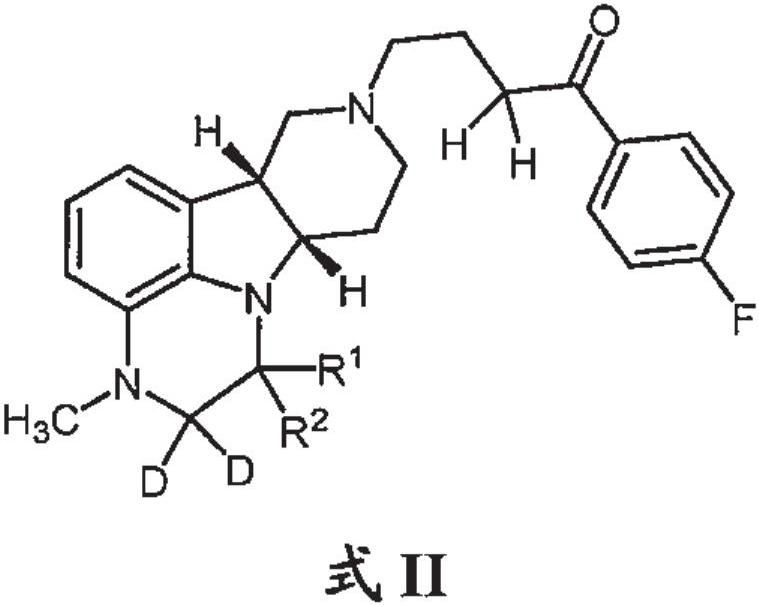
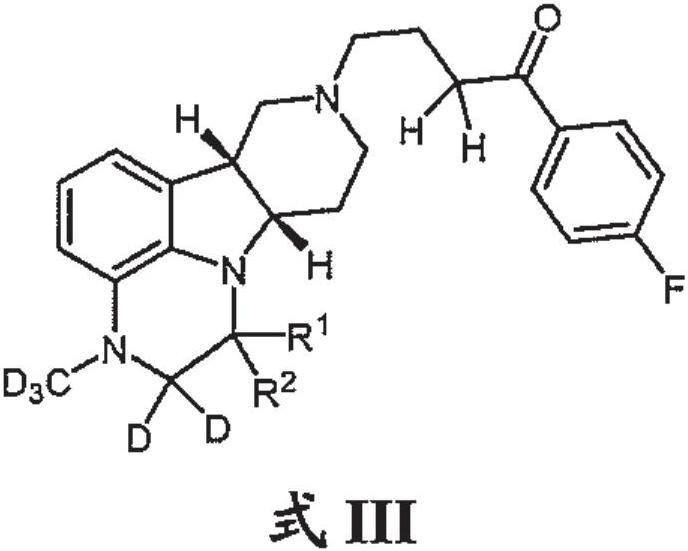
Organic compounds
A technology of compound and composition, applied in the direction of organic chemistry, organic chemical method, heterocyclic compound isotope introduction, etc., can solve problems that have not been fully elucidated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] 1-(4-fluorophenyl)-4-((6bR,10aS)-1,1,2,2-tetradeutero-3-methyl-2,3,6b,7,10,10a-hexahydro -1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxalin-8(9H)-yl)butan-1-one p-toluenesulfonate
[0220]
[0221] Step 1: Addition of degassed 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylic acid (4aS, 9bR)- Ethyl ester (1.60 g, 8.0 mmol), 2-chloro-2,2-dideutero-N-methylacetamide (1.74 g, 16 mmol) and KI (2.68 g, 16 mmol) in dioxane (28 mL) Diisopropylethylamine (2.8 mL, 16 mmol) was added to the mixture. The reaction mixture was then heated to 104 °C for 20 h with vigorous stirring. The solvent was removed in vacuo, the residue was suspended in dichloromethane (50 mL) and extracted with water (20 mL). Separate the organic phase with K 2 CO 3 Dry and concentrate to a residue. The product was purified by column chromatography using a 0-100% mixed solvent gradient in ethyl acetate [ethyl acetate / methanol (10:1 v / v)] to give 6-bromo-5-(1,1-bis Deutero-2-(methyla...
Embodiment 2
[0227] Example 2: Determination of maternal and metabolite levels in mice
[0228] The compound of Example 1 and the compound of formula Q were co-administered in mice (n=3), and the levels of these two compounds were studied. Synthesis of compounds of formula Q can be found in WO 2008 / 112280. Plasma and brain levels were determined at 0.25, 0.5, 1, 2 and 4 hours after single oral dose administration of test compounds. The mean values of the maximum concentration, time to maximum concentration and area under the curve (AUC) of the two compounds were determined. The results are summarized in Table 1 below.
[0229]
[0230] Both plasma and blood concentrations of the compound of Example 1 were found to be higher than those of the compound of formula Q, resulting in higher Cmax values and higher AUC values. This indicates that the tetradeuterated compound of Example 1 has reduced metabolic clearance compared to its non-deuterated counterpart, the compound of Formula Q....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com