Tumor neoantigen polypeptide and application thereof
A technology of antigens and variants, applied in the direction of fusion peptides, anti-tumor drugs, cancer antigen components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The preparation methods of the above TCR and antibody are known to those skilled in the art, including but not limited to, expressing and purifying from Escherichia coli cells or insect cells.
[0112] The TCR and / or antibody may be provided to the cell surface, such as the TCR to the surface of a T cell. Therefore, the present invention also provides an isolated monoclonal T cell that binds to the polypeptide-MHC complex of the second aspect of the present invention.
[0113] The present invention also provides the use of the polypeptide and its variant, polypeptide-MHC complex, tandem polypeptide, cell, nucleic acid molecule, molecule or T cell, for preparing a drug for preventing or treating cancer.
[0114] The present invention also provides a pharmaceutical composition, which contains a pharmaceutically acceptable carrier and the peptide, polypeptide-MHC complex, tandem polypeptide, cell, molecule or T cell.
[0115] The pharmaceutical composition is suitable for...
Embodiment 1
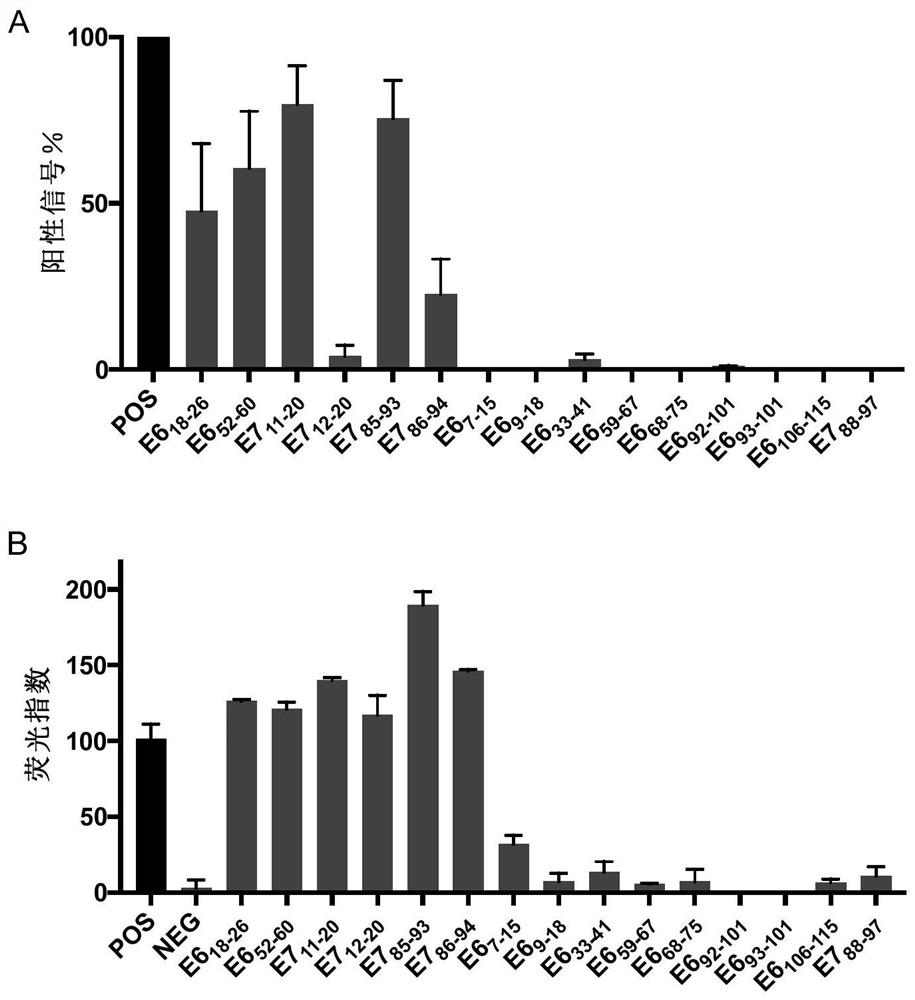
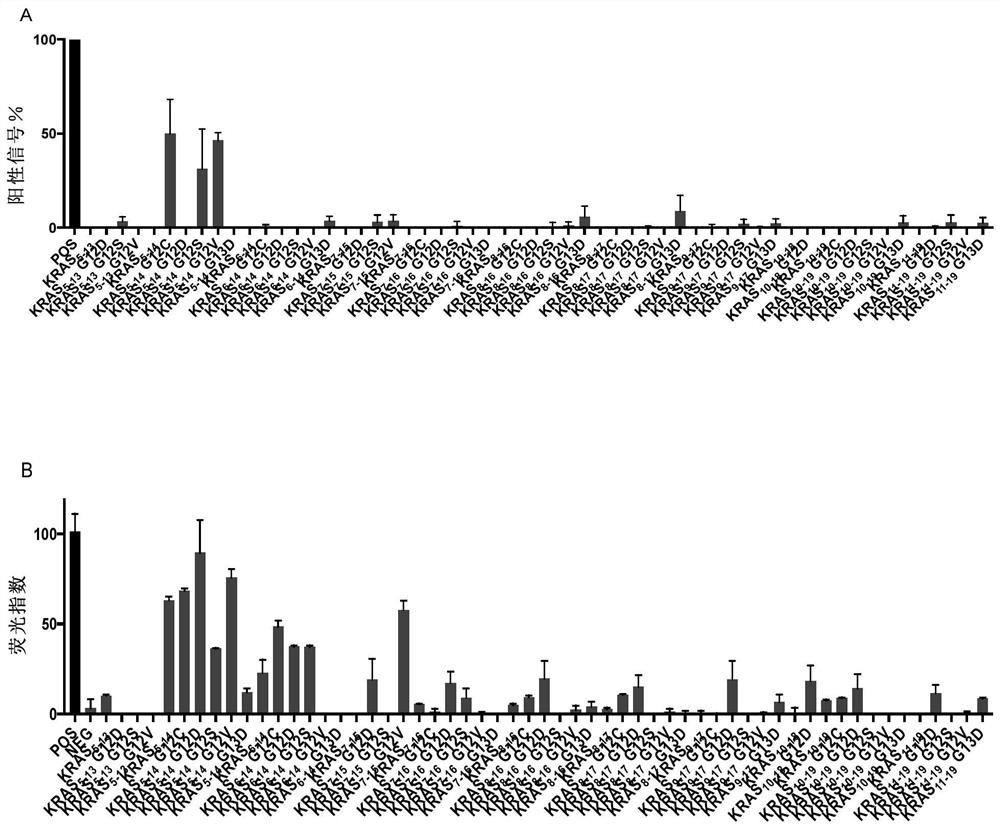
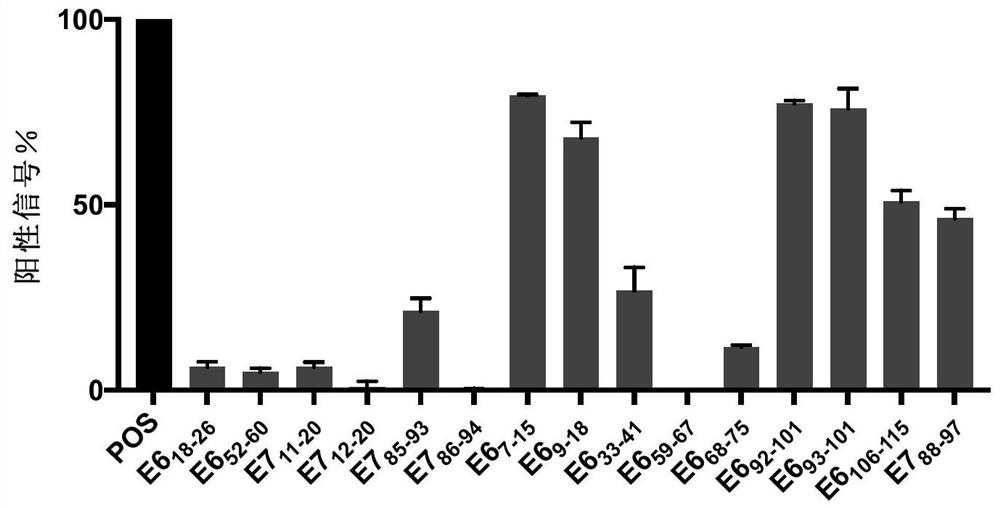
[0122] Example 1. HLA-A2 and HLA-A11 high affinity KRAS and HPV16 polypeptides.
[0123] First use the established human papillomavirus T cell antigen database (HPVdb) (Zhang, G.L., et al., HPVdb: a data mining system for knowledge discovery in human papillomavirus with applications in T cell immunology and vaccinology. Database (Oxford), 2014.2014: p.bau031.) and promiscuous MHC-binding peptide prediction server to predict HPV16- and KRAS-derived peptides with high affinity to HLA-A2 and HLA-A11, respectively. The present invention predicted 15 HPV16-derived polypeptides (Table 1), which showed binding ability to HLA-A02 and HLA-A11.
[0124] Table 1 Peptides derived from HPV16
[0125] gauge sequence SEQ ID No.: HPV16 E6 7-15 (E6 7-15 )
AMFQDPQER 1 HPV16 E6 9-18 (E6 9-18 )
FQDPQERPRK 2 HPV16 E6 18-26 (E6 18-26 )
KLPQLCTEL 3 HPV16 E6 33-41 (E6 33-41 )
IILECVYCK 4 HPV16 E6 52-60 (E6 52-60 )
FAFRDLCIV 5 ...
Embodiment 2
[0131] Example 2. Polypeptide UV exchange analysis and HLA-ⅠELISA detection
[0132]These predicted peptides were custom-synthesized by Elim Biopharmaceuticals and Jill Biochemical Shanghai Company with a purity of >80%. Synthetic polypeptides were resuspended to 10 mM in deionized water and stored at -80°C.
[0133] Prepare 50 μM and 25 μM polypeptides, 0.025 mg / mL HLA-A02:01 monomer and 0.0125 mg / mL HLA-A11:01 monomer by dilution in 1×PBS. Mix 50 μM peptide and 0.025 mg / mL HLA-A02:01 monomer containing UV-sensitive peptide, or 25 μM peptide and 0.0125 mg / mL HLA-A11:01 monomer containing UV-sensitive peptide in equal volumes on ice. The mixture was incubated under 366nm ultraviolet light exposure for 30 minutes, the UV-sensitive peptides on the human MHC class I molecules were destroyed, the peptide binding sites of the human MHC class I molecules were exposed, and the co-incubated target peptides could bind to the human HLA groove , the HLAⅠmolecular monomer that is not bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com