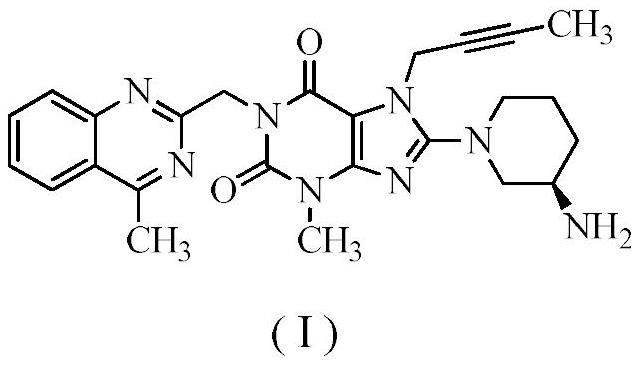
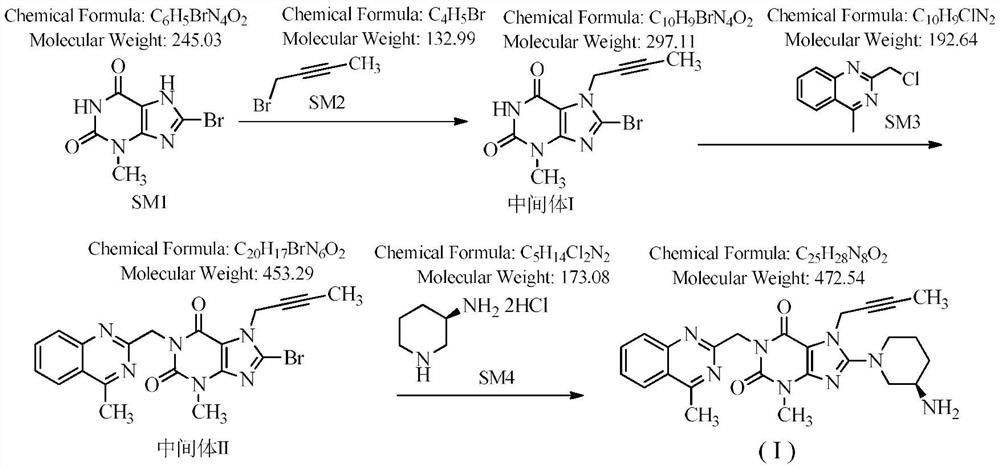
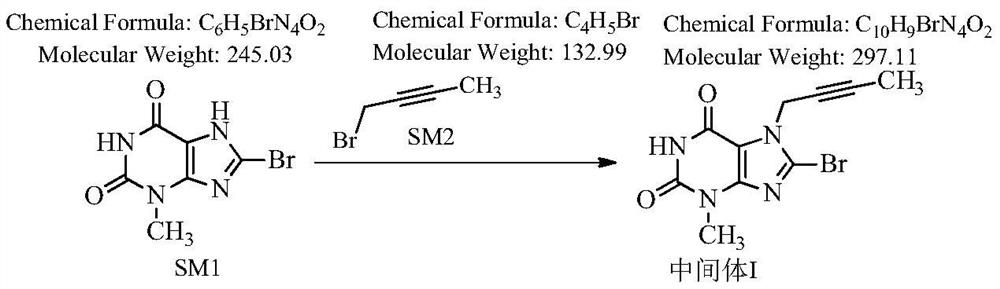
Novel preparation process of linagliptin
A one-pot, reaction technology, applied in metabolic diseases, organic chemistry, drug combination, etc., can solve the problems of three wastes, high cost, and few varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Step 1: Preparation of 8-bromo-7-(2-butyne)-3-methyl-1H-purine-2,6(3,7-dihydro)-dione
[0045] 100 g 8-bromo-3-methylxanthine, 57 g 1-bromo-2-butyne (1.05 eq), 900 ml DMF, 63.3 g DIPEA (1.2 eq) were added to the reaction flask. Stir at room temperature, TLC detection, complete reaction in 18 hours, and an off-white solid is obtained.
[0046] Step 2: Preparation of 8-bromo-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methanol base]-1H-purine-2,6-dione
[0047] Add 82.5 g of 2-chloromethyl-4-methylquinazole (1.05 eq) to the reaction flask of the first step above, stir at 60-70° C., detect by TLC, the reaction is complete in 13 hours, and it is directly used in the next step.
[0048] Step 3: Preparation of Linagliptin
[0049] Add 74.2g of (R)-3-aminopiperidine dihydrochloride (1.05eq) to the reaction flask of the second step above, continue to stir at 60-70°C, detect by TLC, and the reaction is complete in 7 hours. Evaporate the solvent under red...
Embodiment 2
[0051] Different from Example 1: the catalyst triethylamine is 49.6g (1.2 equivalents), the total yield of the three-step reaction is 76.3%, and the HPLC purity is 99.68%.
Embodiment 3
[0053] Different from Example 1: the amount of DIPEA is 68.6g (1.3 equivalents), the total yield of the three-step reaction is 77.9%, and the HPLC purity is 99.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com