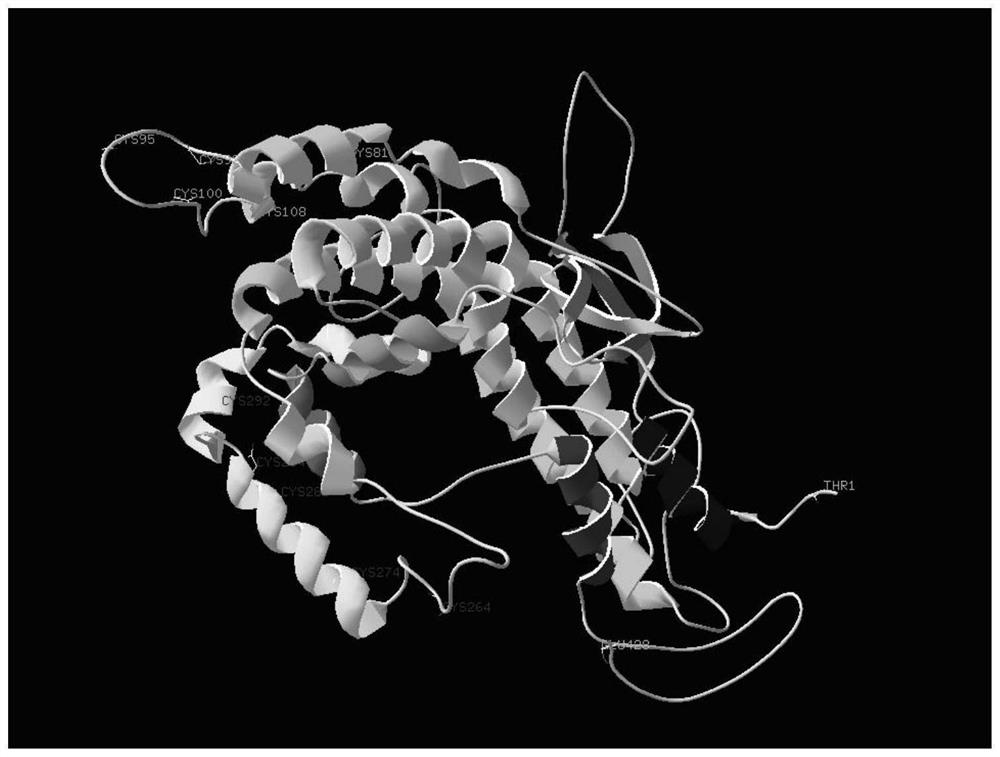
Crystal structure and application of recombinant apolipoprotein J and analogues thereof
Apolipoprotein domain technology, applied in the field of preparation of recombinant apolipoprotein J, can solve the problem of limited source of apolipoprotein J, can not be used in pharmaceuticals, etc., achieve obvious immunological activity, good application value, not easy to immunize The effect of the primordial reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Extraction of Recombinant Apolipoprotein J
[0038] 1.1 Bacterial fragmentation
[0039] (1) Sufficiently resuspend each 1g of bacteria (including recombinant SEQ ID.NO10 sequence) with 10mL suspension solution, centrifuge at 8000rpm, 4°C for 15min, and discard the supernatant; (2) Resuspend with 10mL / g suspension solution again Suspend the bacteria, place the bacteria suspension under the probe of the ultrasonic breaker, power: 400W, ultrasonic 3s, intermittent 6s, and the total time is 15min to break until the break is complete. The construction of the bacterium is a prior art, and will not be repeated here. Preferably, the suspension solution: 50mM Tris-HCl, pH 8.0.
[0040] 1.2 Inclusion body washing
[0041] (1) Centrifuge the crushed bacteria at 15000g at 4°C for 30nim, discard the supernatant, wash with washing solution, add 10mL of washing solution per gram of bacteria, resuspend fully after adding the washing solution, and shake at 4°C Incubate fo...
Embodiment 2
[0057] APO-J (concentration 0.3 mg / mL) corresponding to the partial sequence (SEQ ID. NO3, 4, 7, 11, 12, 15, 16, 18, 19, 22, 23, 26, 27) after SEC chromatography SEC-HPLC detection was carried out, and the results are shown in Table 1. The detection conditions are: TSKGel chromatographic column (300mm×7.8mm), packing particle size 5μm; the experimental instrument is Agilent 1200-DAD GR11010670, the mobile phase is 100mM PBS+200mM urea, pH 6.7; the injection volume is 80μl; the flow rate is 0.7ml / min .
[0058] Table 1 The purity of APO-J corresponding to different sequences by SEC chromatography
[0059] corresponding sequence Sample purity, % Impurities, % corresponding sequence Sample purity, % Impurities, % SEQ ID. NO3 94.13 5.86 SEQ ID. NO18 96.12 3.85 SEQ ID. NO4 94.67 5.31 SEQ ID. NO19 95.21 4.76 SEQ ID. NO7 95.11 4.87 SEQ ID. NO22 94.97 5.01 SEQ ID. NO11 93.91 6.05 SEQ ID. NO23 94.38 5.58 SEQ ID. NO12...
Embodiment 3
[0061] Example 3 Optimizing the renaturation process
[0062] 3.1 Effect of different renaturation processes on sample stability
[0063] Experimental procedure: Take 6ml of the sample after cation exchange chromatography in Example 1, add different refolding buffers at a volume ratio of 1:16, add to the sample, incubate at 4°C for 4h, concentrate directly or desalt to 20mM PB , concentrated to 3-5mg / ml after pH 7.4, incubated with 3 times the mass of SKL at room temperature for 16 hours and passed Superdex 200pg, the mobile phase was 10mM PB, 200mM Na-citrate, 1% SKL, pH7.4. Place the sample at 4°C, 37°C, and RT (room temperature), take samples on day 0, day 1, day 2, day 3, and day 4, inject 0.5ml, and measure the stability of the sample. The results are shown in Table 3, Table 4. At the same time, each group of samples was repeatedly frozen and thawed 1, 2, 3, and 4 times, and the purity of the samples was measured after each freezing and thawing; the repeated freezing an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com