Long noncoding RNA (lncRNA) for clinical risk assessment of preeclampsia (PE) and application of lncRNA
A clinical risk assessment, long-chain non-coding technology, applied in the medical field, can solve problems such as few drugs and heavy economic burden, and achieve strong feasibility and simple and convenient sample acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The technical scheme of the present invention will be described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0036]The long-chain non-coding RNA used for clinical risk assessment of preeclampsia, the specific implementation steps are as follows:
[0037] 1 Material preparation
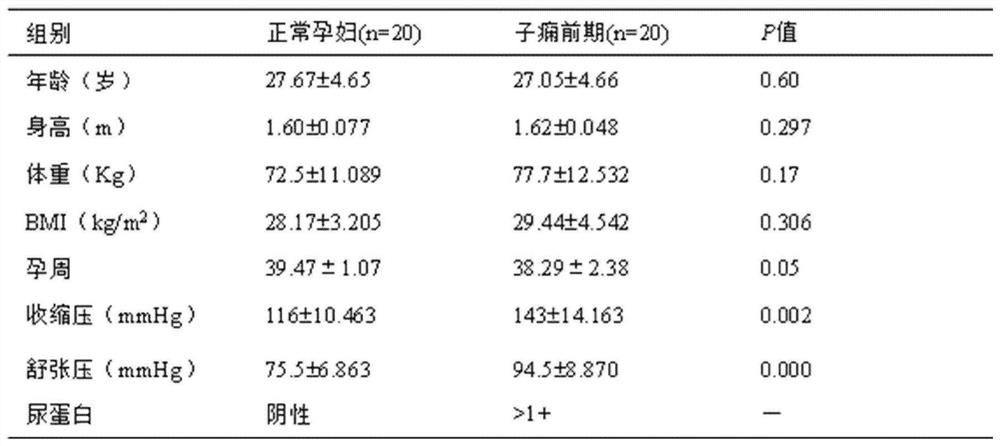
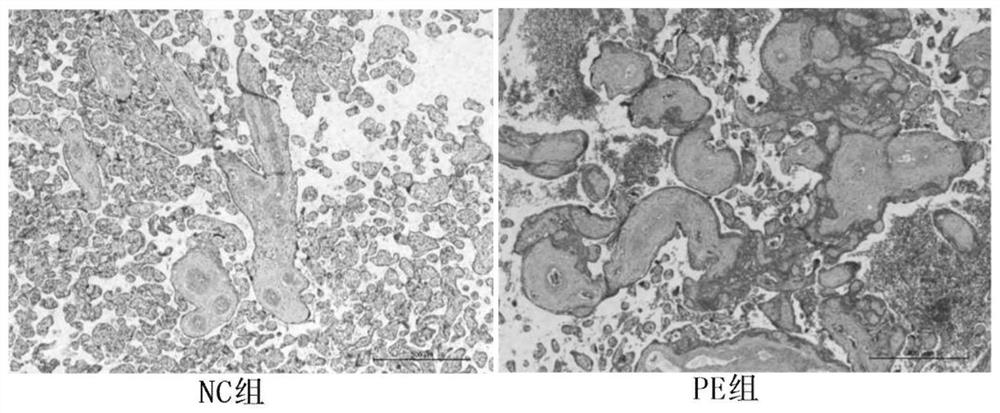
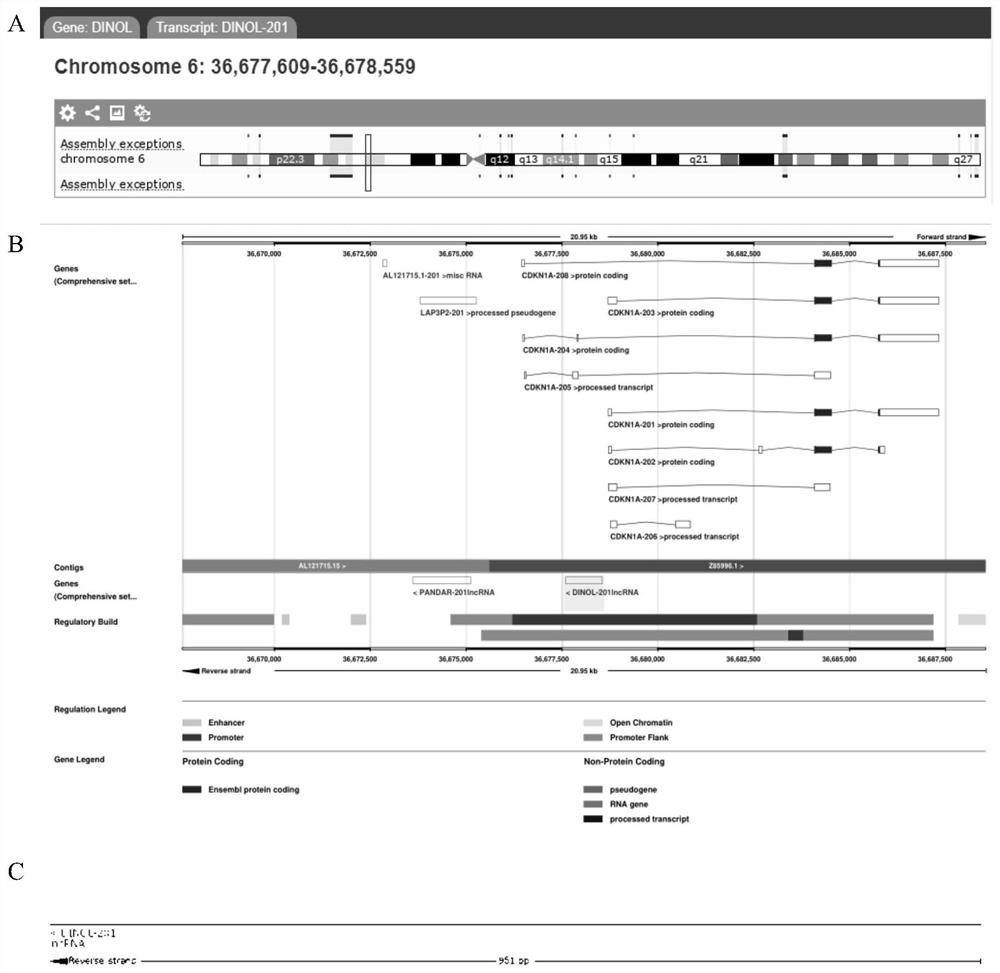
[0038] 1.1 Materials and reagents: Human placental trophoblast cell line HTR-8 / SVneo (human chorionic trophoblast cells) (China, Shanghai Ruilu Biotechnology Co., Ltd.); fetal bovine serum, RPMI 1640 medium (Australia, Gibco company) ; penicillin, streptomycin, trypsin digestion solution, NP-40 (China, Beyontian Institute of Biotechnology); Masson staining kit, PMSF (China, Beijing Suolaibao Technology Co., Ltd.); MMP-2 (matrix metalloproteinase 2) Antibody (UK, Abcam Company), β-actin (β-actin) antibody, horseradish peroxidase-labeled secondary antibody (China, Beijing Jinqiao Co., Ltd.); skimmed milk powder (USA, BD Company); cDNA kit, RT-qPCR (re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com