A kind of sericin peptide and its application
A technology of sericin and protein peptide, applied in the field of biomedicine, can solve the problems of reporting the function of sericin inflammatory factors, and achieve the effect of inhibiting inflammatory factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, the extraction of sericin
[0017] A method for extracting sericin, comprising the steps of:
[0018] (1) clean the silkworm cocoons with water;
[0019] (2) Cut into cocoon pieces with a size of 2*2cm, and heat them in an oven at 120°C for 2.5 hours;
[0020] (3) Add Milli-Q water according to the bath ratio of 1:30, and treat at 120°C for 1h and 2h respectively;
[0021] (4) filter cocoon sheet residue, collect protein extract;
[0022] (5) Freeze-dry the protein extract to make a powder, and store it in a -20°C refrigerator.
[0023] The sericin protein obtained by high temperature treatment for 1 h is coded as HS1, and the sericin protein obtained by high temperature treatment for 2 h is coded as HS2.
Embodiment 2
[0024] Embodiment 2, sericin protein anti-inflammatory effect
[0025] 1. Cell culture method:
[0026] Cells were cultured at 37°C, 5% CO 2 In a constant temperature incubator, NIH3T3 and HaCaT cell lines were cultured with DMEM medium containing 10% fetal bovine serum, and RAW264.7 cell lines were cultured with 1640s medium containing 10% fetal bovine serum. All cell lines were changed medium every day and subcultured every other day.
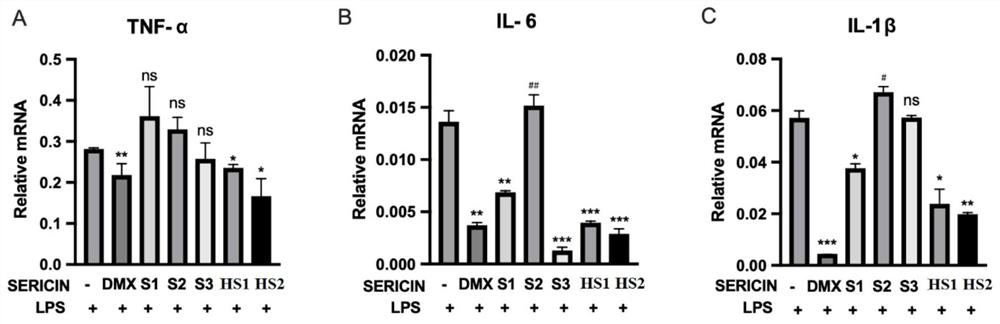
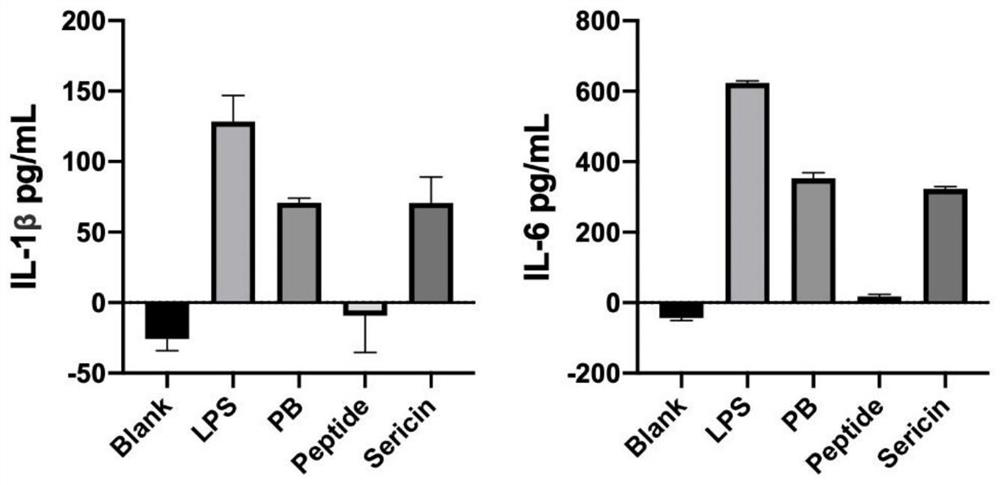
[0027] Three kinds of cells were treated with HS1 and HS2 prepared in Example 1, and three commercially available sericin proteins (numbered S1, S2, S3) were used as controls, and then inflammatory factors were detected by real-time quantitative PCR and ELISA.
[0028] 2. ELISA detection
[0029] (1) After the cells are processed, absorb the cell culture medium, centrifuge at 13,500 rpm for 10 minutes, and take the supernatant; use it now or store it at -20°C for later use.
[0030] (2) Take a microwell plate, add 100 μL of cell culture s...
Embodiment 3
[0073] Embodiment 3, sericin protein hydrolysis and anti-inflammatory effect
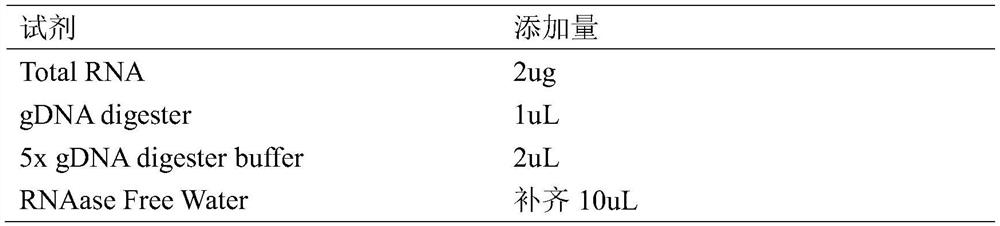
[0074] In order to understand the function of the polypeptide after hydrolysis of sericin protein, LC-MS / MS detection is performed after hydrolysis of sericin protein. The detection method of LC-MS / MS is as follows:
[0075] Using the FASP enzymatic hydrolysis method, the process is carried out according to enzymatic hydrolysis-desalting-mass spectrometry-protein identification, and the specific steps are as follows:
[0076] Enzymolysis:
[0077] (1) Take the protein solution, add DTT with a final concentration of 100mM, heat at 95-100°C for 5min, and cool to room temperature.
[0078] (2) Add 200 μL of UA buffer and mix well. Transfer to a 30kD ultrafiltration centrifuge tube, centrifuge at 14000g for 15min, and discard the filtrate.
[0079] (3) Repeat step (2).
[0080] (4) Add 100 μL of 100 mM IAA buffer solution, shake at 600 rpm for 1 min.
[0081] (5) Place at room temperature, protect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com