Dimeric immunofusion proteins, pharmaceutical compositions and uses
A fusion protein and dimer technology, applied in the direction of drug combination, fusion polypeptide, peptide/protein components, etc., to achieve the effect of reducing tissue damage, good therapeutic effect, and broad clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Construction, expression and characterization of soluble dimer immunofusion proteins
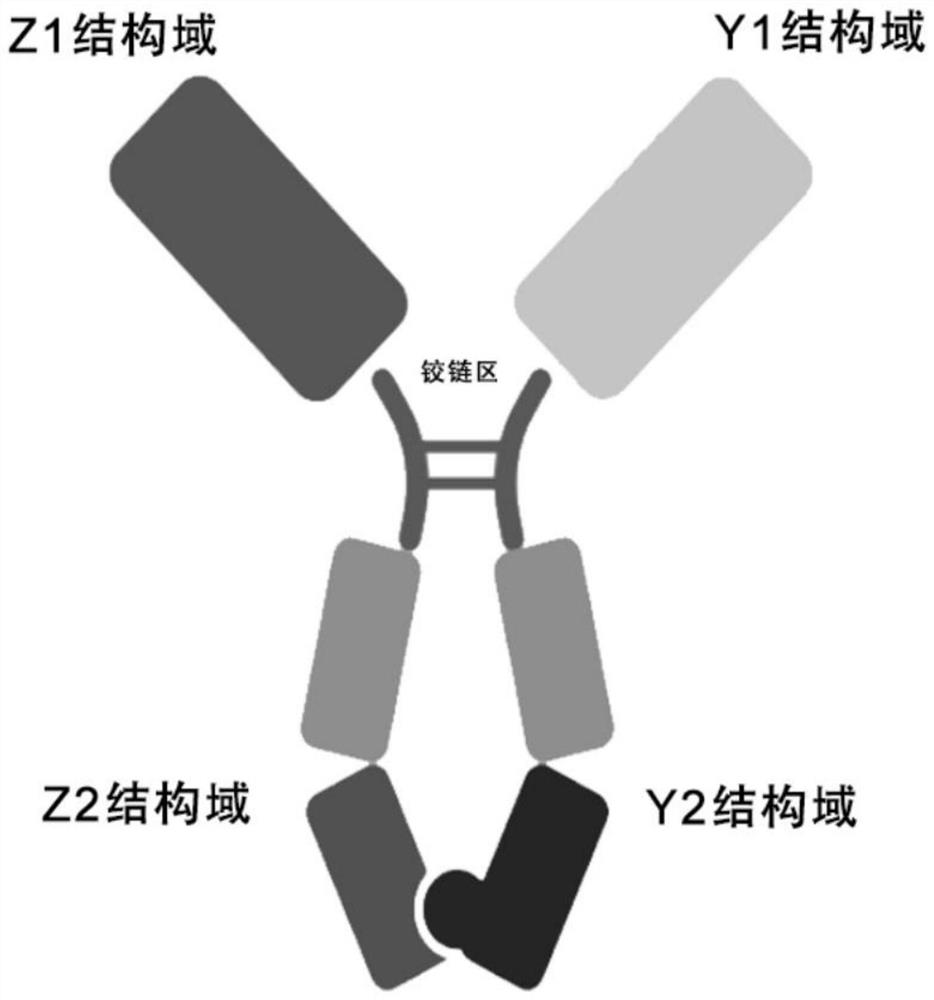
[0076] like figure 1 As shown, the soluble dimer immunofusion protein is a dimer with antibody Fc, and the construction and expression methods of the dimer immunofusion protein itself are routine experimental techniques in the field, which are briefly described as follows:
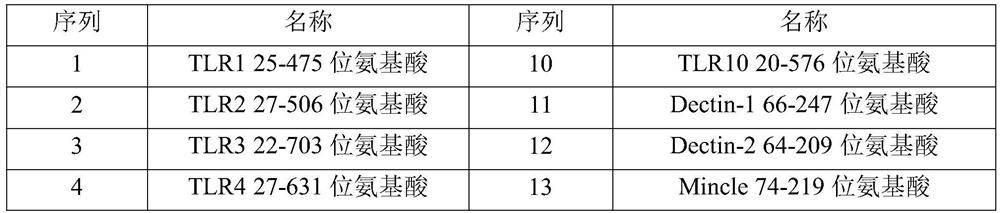
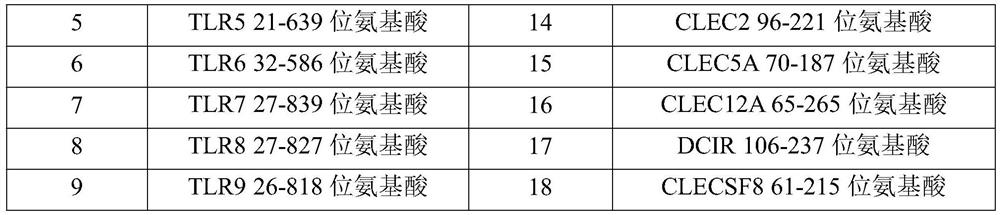
[0077] (1) Entrust a gene synthesizer (Suzhou Jinweizhi Company) to perform coding nucleotide codon optimization and whole gene synthesis for the amino acid sequence of the dimer immune fusion protein required in this example, and the optimized nucleotide sequence is directly loaded into the On the PCDNA3.4 vector, the amino acid sequences encoded by all vectors are described in Table 1.
[0078] (2) Entrust a protein manufacturer (Yiqiao Shenzhou Company) to express and purify a dimer immunofusion protein for this example. Using the literature Finck B K. Science, 265.; Mihara M et al.. Journal of Cl...
Embodiment 2 2
[0088] Example 2 Effect of dimer immunofusion protein on Staphylococcus aureus
[0089] Staphylococcus aureus expressing green fluorescent protein was collected from the Institute of Microbiology, Chinese Academy of Sciences, and the multiplicity of infection was 1:10. Peripheral blood samples were collected from healthy volunteers to isolate peripheral mononuclear cells (PBMCs, which were isolated using Biyuntian Lymphocyte Separation Kit). Freshly isolated cells were stabilized for 2 h in RPMI1640 medium containing 10% fetal bovine serum (37.5°C, 5% CO). 2 ).
[0090] Cultivate Staphylococcus aureus according to the experimental requirements, collect the bacteria by centrifugation at 10000g for 30 seconds in a tabletop centrifuge, and resuspend to 10 8 Bacterial density around CFU / ml. The bacterial suspension was taken for serial dilution plate counts to determine the exact bacterial density. After the PBMC cells were replaced by medium, 10 microliters of PBS resuspended...
Embodiment 3
[0098] Example 3 Effect of immunodimer on methicillin-resistant Staphylococcus aureus
[0099] BALB / c mice, SPF grade, female, 6-8 weeks old, weighing 18-20 g, international standard strain MRSA-252, purchased from American Tissue Culture Collection (ATCC). A mouse model was established, and 0.1 mL of washed bacterial solution (the concentration of bacterial solution was 1 × 10) was injected through the tail vein. 9 CFU / mL), mice in the blank group were injected with an equal volume of sterile normal saline through the tail vein. Then the mice were divided into a control group and a treatment group, with 10 mice in each group, the control group was given control IgG, and the treatment group was given a representative of the dimer immunofusion protein of the present invention at a dose of 10 mg / kg, intravenously Injection once a day, continuous observation for 10d. If the mice died or all the mice were sacrificed at the end of the experiment on the last day, the blood was imm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com