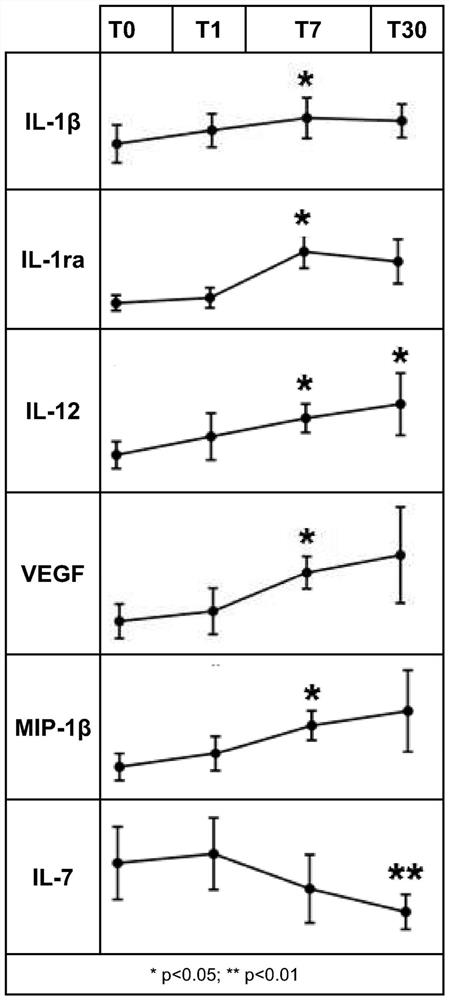
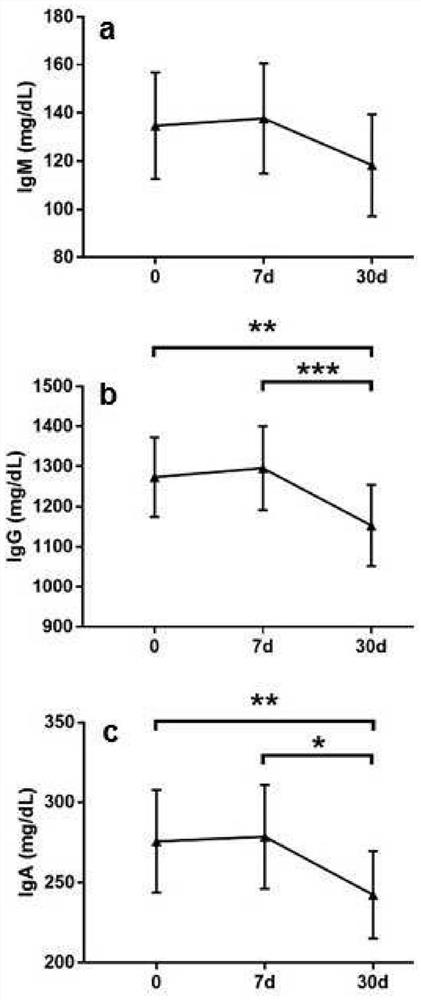
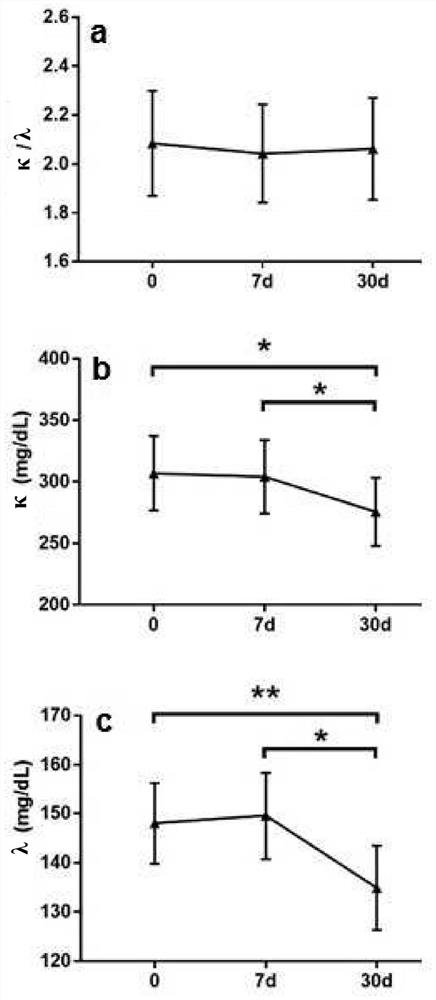
Polidocanol for use as immunomodulating agent
A technology for polidocanol and immune regulation, which is applied in the field of immune and inflammatory response regulators, and can solve problems such as unproven useful effects of polidocanol, improvement of skin nutrition, and undisclosed polidocanol.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Functional injection therapy (FIT) with diluted polidocanol.
[0054] commercially available polidocanol solution with normal saline solution Dilute to 0.05%. Then 1.4% sodium bicarbonate solution was added to obtain a final concentration of 0.06%. Eighty percent of the patients were women between the ages of 18 and 82 with mild to severe venous insufficiency who were treated with their free and informed consent.
[0055] Prior to treatment, approximately 100 patients were surveyed to assess their quality of life and to assess possible changes in inflammatory / inflammatory general status, venous microcirculation and circulatory function, and also more generally skin nutrition.
Embodiment 2
[0057] Example 2. Evaluation of long-term systemic effects of diluted polidocanol: QoL investigation.
[0058] Long-term systemic effects were assessed by surveys administered to patients after 3 or 4 treatments with diluted polidocanol.
[0059] In particular, they were required to be based on the definition in Launois R. et al., "Construction and validation of aquality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ), Qual. Life Res., 1996, 5(6):539-554 Standard fill out questionnaire (CIVIQ-20).
[0060] The questionnaire contained the questions outlined below, with answers indexed from 0 to 4 (0 if the discomfort described by the question was not applicable, and 1 to 4 if increasing levels of intensity were applicable). Below is a collection of activities that were found to improve after treatment:
[0061] 1) In the past 4 weeks, have you had hip or leg pain and to what extent? In 14% of cases a reduction in hip pain was observed after treatment...
Embodiment 3
[0084] Example 3. Likert Questionnaire Regarding the "Inflammation" Status of Treated Subjects
[0085] To the question on quality of life, 10 more specific questions were added to assess possible changes in "inflammatory" status (n=100 patients) after treatment. The survey was based on the indications of: "Evaluation by Likert-type scale 4-point (0-absent, 1-moderate, 2-severe and 3-very severe)" Elleuch et al., Adv Ther .2016Sep;33(9):1536-49.Subjects were asked to conduct a self-assessment after each treatment and to evaluate the survey results after 8 to 10 months (ie, after at least 4 to 5 treatments).
[0086] The survey was customized on a Likert scale and aimed to evaluate a number of symptoms associated with chronic inflammation, such as joint or arthritic manifestations (general and autoimmune sex), which specifically involves joint mobility and pain.
[0087] Answers are indexed on a scale from 0 to 3 (or 0 to 2).
[0088] 1. Joint Mobility
[0089] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com