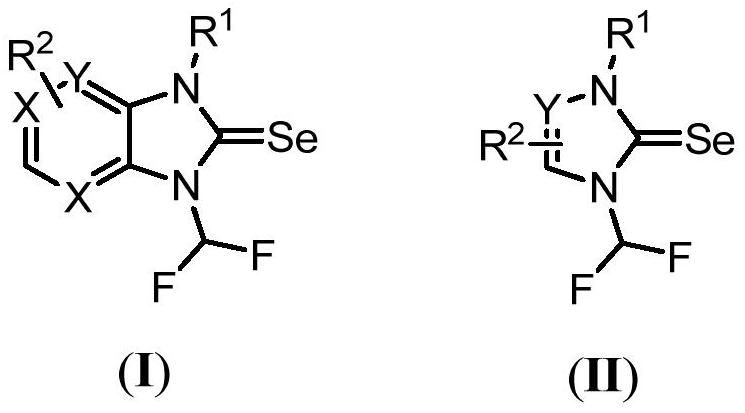
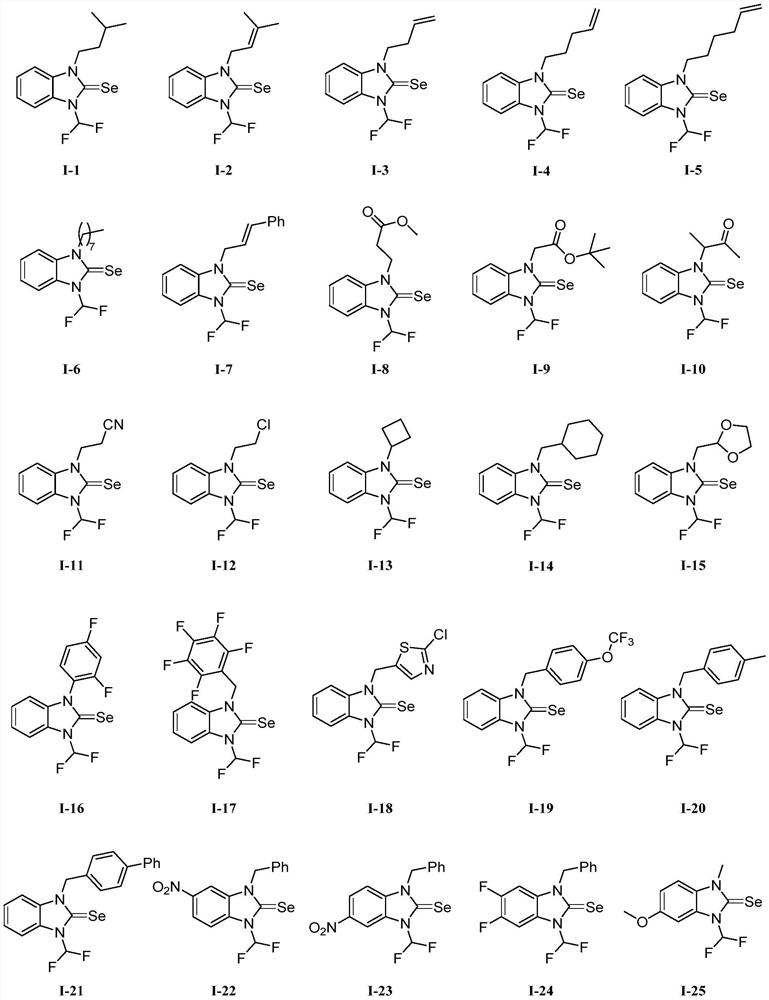
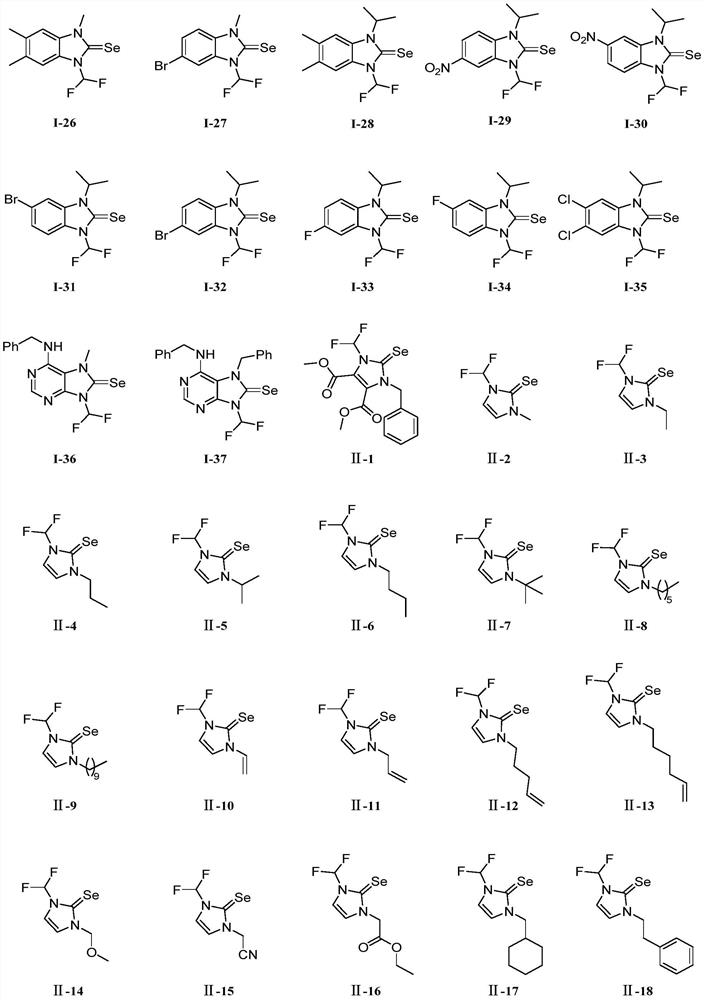
N-difluoromethyl azole selenourea derivative or pesticide acceptable salt and application thereof
A technology of difluoromethyl azoles and derivatives is applied in the field of agricultural pesticides, and can solve the problems of N-difluoromethyl azole selenourea derivatives not being used for insecticidal purposes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0160] Example 3: Evaluation of some compounds on the insecticidal activity of diamondback moth
[0161] The tested insects were Plutella xylostella of Lepidoptera, and the sensitive strains were kept indoors. The middle and late 3rd instar larvae of Plutella xylostella were used as the test object, and the test method was the leaf dipping method.
[0162] Operation process: Accurately weigh each sample, add acetone to prepare 10g / L mother liquor, and dilute it to 500ppm with Tween 80 aqueous solution containing 0.5‰. Use a hole puncher with a diameter of 1.0 cm to make clean cabbage leaves into discs and immerse them in the liquid medicine, take them out after 10 seconds, dry them naturally, and put them into clean containers. Inject middle and late 3rd instar larvae of Plutella xylostella with the same growth into the container, and insert 10 test larvae into each treatment, and raise them at a constant temperature of 28°C. Three repetitions were set, and the control group...
Embodiment 4
[0163] Example 4: Evaluation of the insecticidal activity of some compounds against Spodoptera frugiperda
[0164] The tested insects were Spodoptera frugiperda of Lepidoptera, and the sensitive strains were kept indoors. The end-2nd instar larvae of Spodoptera frugiperda were used as the test object, and the test method was the leaf dipping method.
[0165] Operation process: Accurately weigh each sample, add acetone to prepare 10g / L mother liquor, and dilute it to 500ppm with Tween 80 aqueous solution containing 0.5‰. Use a hole puncher with a diameter of 1.0 cm to make clean corn leaves into leaf disks and immerse them in the liquid medicine, take them out after 10 seconds, let them dry naturally, and put them into clean containers. Insert the end-2nd instar larvae of Spodoptera frugiperda with the same growth into the container, insert 10 test worms into each treatment, and raise them at a constant temperature of 28°C. Three repetitions were set, and the control group wa...
Embodiment 5
[0166] Example 5: Evaluation of the insecticidal activity of some compounds against beet armyworm
[0167] The tested insects were beet armyworm of Lepidoptera, and the sensitive strains were kept indoors. The first instar larvae of beet armyworm were used as the test object, and the test method was the leaf dipping method.
[0168] Operation process: Accurately weigh each sample, add acetone to prepare 10g / L mother liquor, and dilute it to 500ppm with Tween 80 aqueous solution containing 0.5‰. Use a hole puncher with a diameter of 1.0 cm to make clean cabbage leaves into discs and immerse them in the liquid medicine, take them out after 10 seconds, dry them naturally, and put them into clean containers. Inject 1st-instar mid-stage larvae of beet armyworm with uniform growth into the container, and insert 10 test worms into each treatment, and raise them in a constant temperature incubator at 28°C. Three repetitions were set, and the control group was treated with an acetone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com