Quinolinone Schiff base compound, and preparation method and application thereof
A compound and drug technology, applied in the field of pesticides, can solve problems such as the use of quinolinone Schiff base compound pesticides and vegetable seed germination accelerators, etc., and achieve excellent insecticidal effect, good poisoning effect, and structure. novel effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation method of said compound specifically comprises the following steps:
[0064] a. Dissolve 2-aminoacetophenone in absolute ethanol, then add NaOH ethanol solution, stir in an ice bath, then add a mixture of compound A and absolute ethanol, and react at 0-5°C;
[0065] b. After the reaction is completed, adjust the pH value of the reaction solution to neutral, then add p-toluenesulfonic acid to the reaction solution, and react at 65-70°C;
[0066] c. After the reaction is completed, pour the reaction solution into ice water, adjust the pH of the solution to 7-9 with triethylamine, and precipitate out, filter, wash until neutral, and then recrystallize with absolute ethanol to obtain the quinolinone intermediate body;
[0067] d. Dissolve the quinolinone intermediate in acetic acid, then add a mixture of cyanoacetylhydrazide and absolute ethanol, and react at 25-35°C. After the reaction is completed, remove the solvent to obtain a solid mixture, and then use ...
Embodiment 1
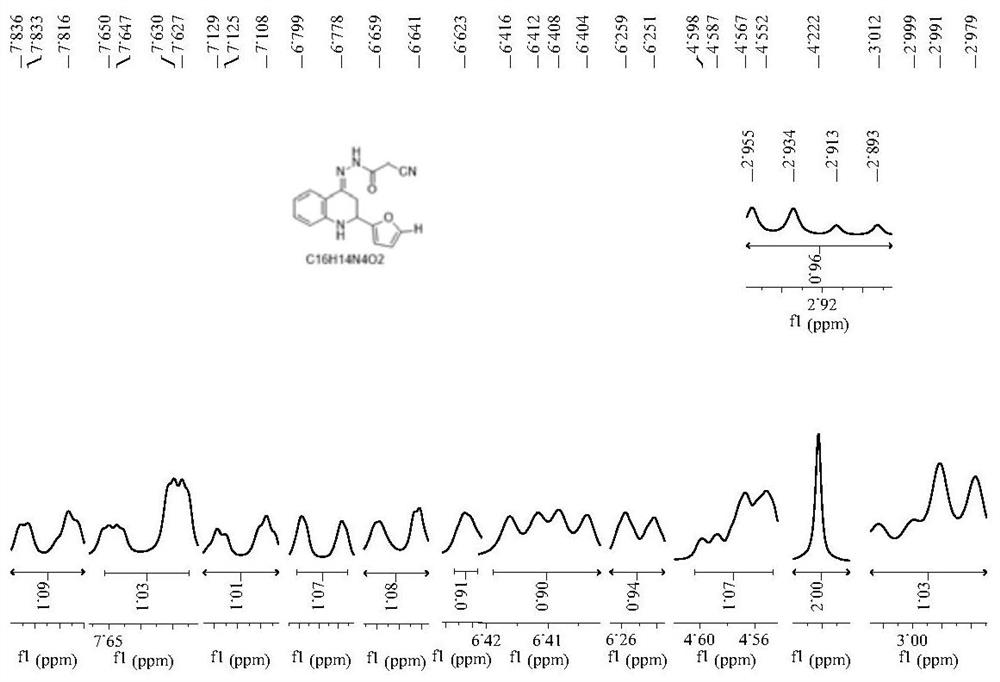
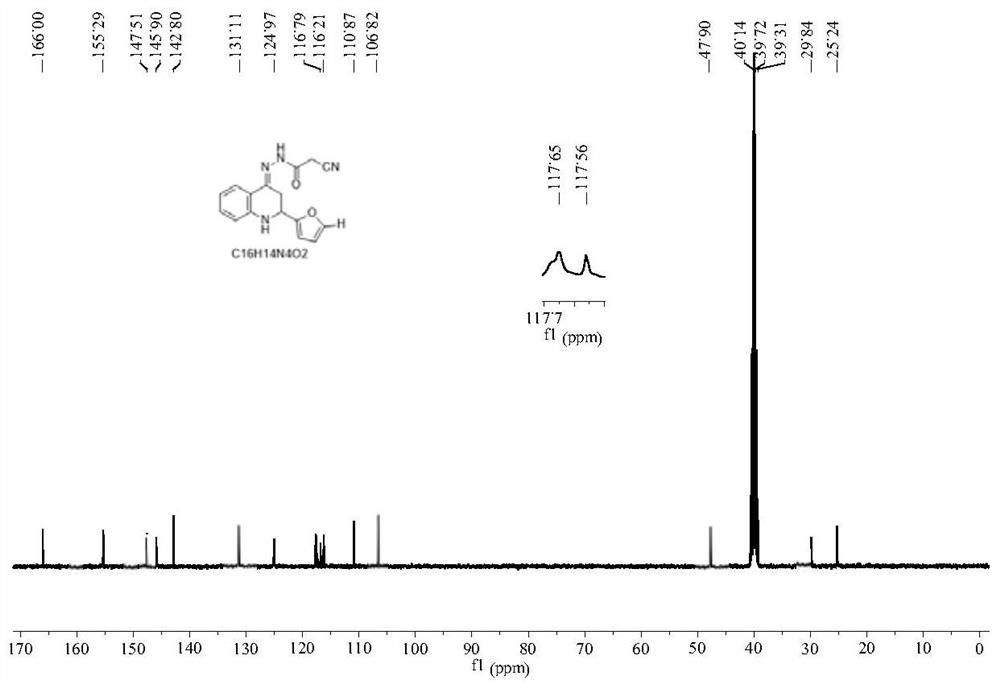
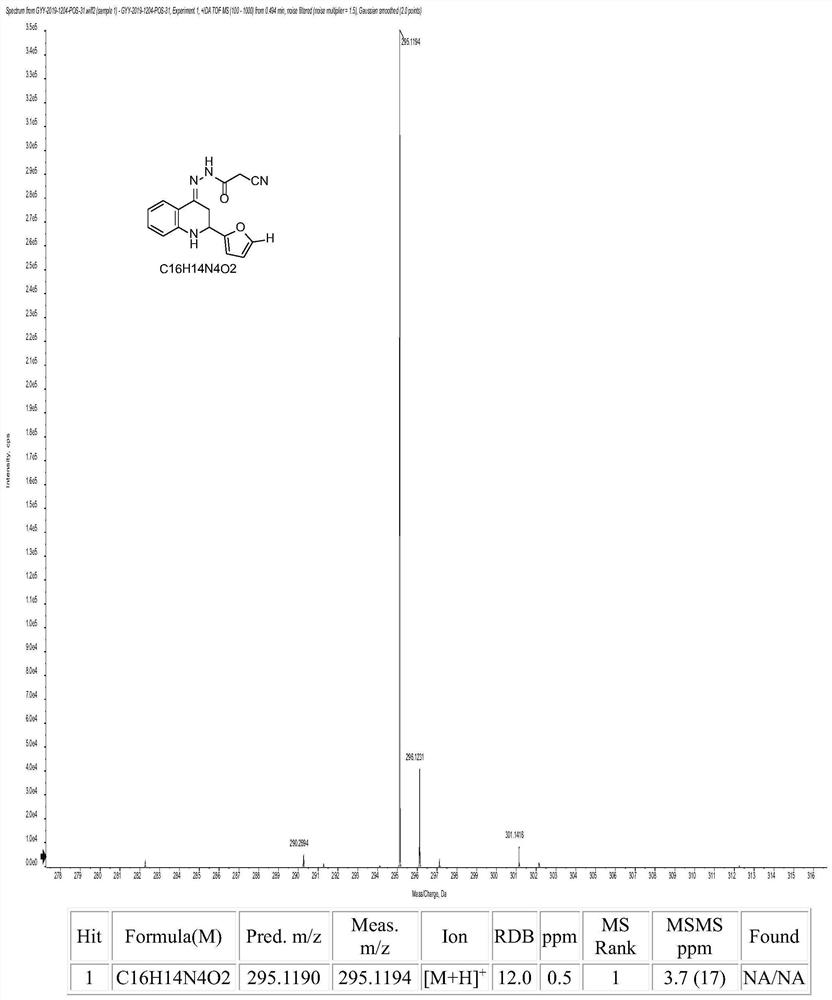
[0079] Compound 1: preparation of
[0080] 0.01 mol of 2-aminoacetophenone was dissolved in 10 mL of absolute ethanol, and 10 mL of 10% NaOH ethanol solution was added thereto. Stir in an ice bath, slowly drop the mixture of 0.01mol furfural and 10mL absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and check with a thin-layer silica gel plate (TLC) whether the reaction is complete. After the reaction was completed, the pH of the solution was adjusted to neutral with 10% HCl. Then add 0.01mol of p-toluenesulfonic acid to the reaction mixture, react at 65-70°C, and check whether the reaction is complete by TLC. After the reaction is completed, pour the reaction solution into 100 mL of ice water, adjust the pH of the solution to 7-9 with triethylamine, filter, wash with distilled water until neutral, and then recrystallize with absolute ethanol to obtain quinolinone intermediate.
[0081] Dissolve 0.01 mol of homemade ...
Embodiment 2
[0084] Compound 2: preparation of
[0085] 0.01 mol of 2-aminoacetophenone was dissolved in 10 mL of absolute ethanol, and 10 mL of 10% NaOH ethanol solution was added thereto. Stir in an ice bath, slowly drop the mixture of 0.01mol 5-methylfurfural and 10mL absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction was completed, the pH of the solution was adjusted to neutral with 10% HCl. Then add 0.01mol of p-toluenesulfonic acid to the reaction mixture, react at 65-70°C, and check whether the reaction is complete by TLC. After the reaction is completed, pour the reaction solution into 100 mL of ice water, adjust the pH of the solution to 7-9 with triethylamine, filter, wash with distilled water until neutral, and then recrystallize with absolute ethanol to obtain quinolinone intermediate.
[0086] Dissolve 0.01 mol o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com