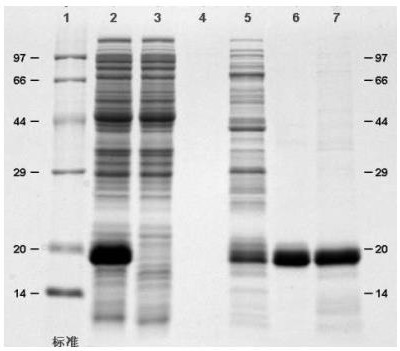
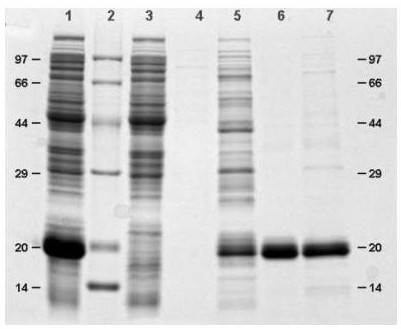
Rapid purification method of recombinant human interferon <alpha>2b
A recombinant human interferon and purification method technology, which is applied in the field of rapid purification of recombinant human interferon α2b, can solve the problems of complex purification process, short production time, and many process steps of recombinant human interferon α2b, and achieve high protein purity, The effect of short production time and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Collect the bacteria produced by fermentation and add 50mM phosphate buffer (PB, pH7.0) at a ratio of 1:15 and stir at room temperature for 2 hours; homogenizer crushed twice, 600bar; use a centrifuge to centrifuge at 14000rpm for 20min, and collect the centrifuged supernatant; The supernatant was filtered using 0.2 μm, and the filtrate was collected.
[0063] Chromatography: flow rate 2ml / min (60cm / hr), CV=35mL, column diameter=26mm
[0064] Chromatography packing material: Ni Sepharose excel (cytiva)
[0065] Buffer: Solution A: 50mM PB+0.15M NaCl (pH 7.5)
[0066] Solution B: 50mM PB+0.15M NaCl+30mM imidazole (pH 7.5)
[0067] Solution C: 50mM PB+0.15M NaCl+100mM imidazole (pH 7.5)
[0068] Solution D: 50mM PB+0.15M NaCl+0.8M imidazole (pH 7.5)
[0069] Solution E: 20% ethanol solution.
[0070] Equilibration: equilibrate 6 times column volume (CV) with solution A,
[0071] Sample loading: Load 400ml of the filtered sample solution,
[0072] Washing: wash 7CV ...
Embodiment 2
[0077] Collect the bacteria produced by fermentation and add 50mM phosphate buffer (PB, pH8.0) at a ratio of 1:10 and stir at room temperature for 2 hours; homogenizer crushed twice, 500bar; use a centrifuge to centrifuge at 14000rpm for 30min, and collect the centrifuged supernatant; The supernatant was filtered using 0.2 μm, and the filtrate was collected.
[0078] Chromatography: flow rate 5ml / min (150cm / hr), CV=35mL, column diameter=26mm
[0079] Chromatography packing material: Ni Sepharose excel (cytiva)
[0080] Buffer: Solution A: 50mM PB+0.15M NaCl (pH 8.0)
[0081] Solution B: 50mM PB+0.15M NaCl+10mM imidazole (pH 8.0)
[0082] Solution C: 50mM PB+0.15M NaCl+60mM imidazole (pH 8.0)
[0083] Solution D: 50mM PB+0.15M NaCl+1M imidazole (pH 8.0)
[0084] Solution E: 20% ethanol solution.
[0085] Equilibration: equilibrate 5 times column volume (CV) with solution A,
[0086] Sample loading: Load 400ml of the filtered sample solution,
[0087] Washing: wash 10CV w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com