Preparation method and preparation device of lithium difluoro(oxalato)borate
A technology of lithium difluorooxalate borate and boron trifluoride, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of low raw material utilization rate, complicated purification process, Problems such as high cost of raw materials, to achieve high product yield, solve the effect of complex purification process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
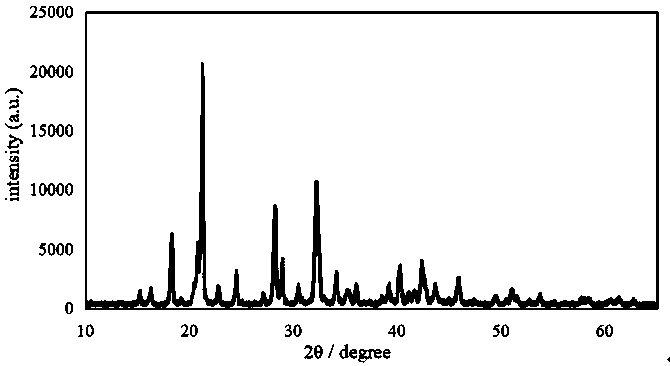
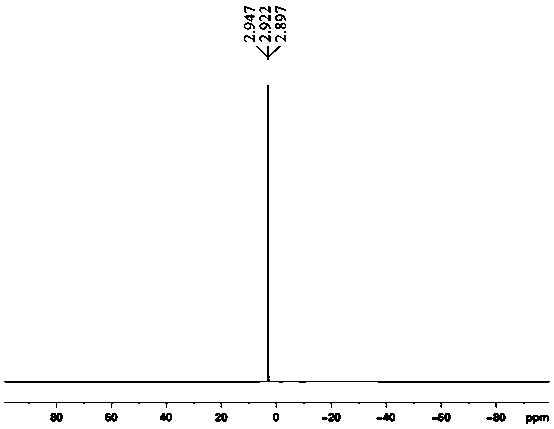
Examples
preparation example Construction
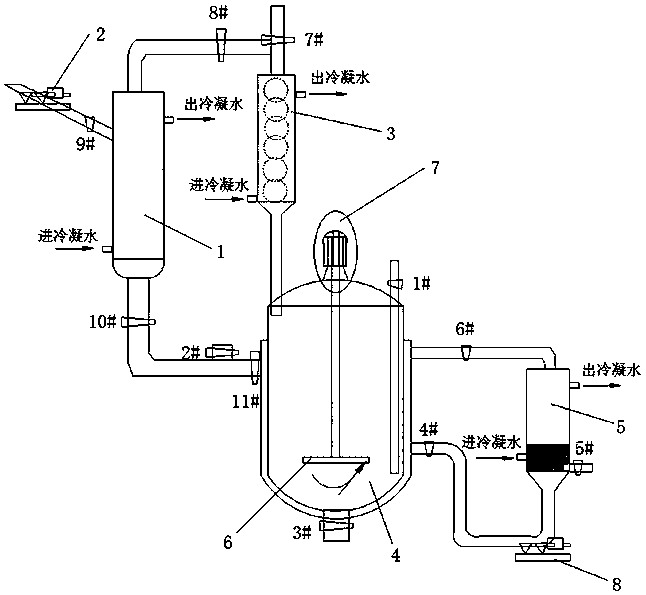
[0035] The invention provides a kind of preparation method of lithium difluorooxalate borate, comprising the steps of:
[0036] 1) Mix boron trifluoride complex, solvent and lithium oxalate, and react at 40-110°C for 2-12 hours to obtain a reaction solution containing lithium tetrafluoroborate and lithium difluorooxalate borate;
[0037] 2) The reaction solution containing lithium tetrafluoroborate and lithium difluorooxalate borate obtained in step 1) is subjected to a vacuum distillation until the amount of solvent is reduced by 1 / 3~1 / 2, and the vacuum distillation is completed to obtain primary distillate and primary distillate;
[0038] 3) Condensing and crystallizing the primary distillation residual liquid obtained in the step 2) to obtain primary crude difluorooxalate lithium borate;
[0039] 4) Mix the primary distillate obtained in step 2) with oxalic acid and a catalyst, react at 35-110°C for 4-8 hours, and then carry out secondary vacuum distillation until the prim...
Embodiment 1
[0065] (1) Open the 2# and 11# valves, add DMC to the constant temperature reactor, the amount added is 1 / 3 of the reactor volume; open the 7# valve, turn on the condensation reflux device, and add BF from the liquid feeding port 2# 3 ·O(CH 2 CH 3 ) 2 (DMC and BF 3 ·O(CH 2 CH 3 ) 2 The molar ratio is 1:2), after stirring, close the 11# valve.
[0066] (2) Open the 1# valve of the solid feeding port, and add Li to the mixed solution 2 C 2 o 4 After closing the valve, its BF 3 ·O(CH 2 CH 3 ) 2 The molar ratio was 1:2; the temperature was raised to 100°C, and the reaction was heated under reflux for 6h.
[0067] (3) Close the 7# valve and the condensing reflux device, open the 8# and 9# valves, start the vacuum distillation device (vacuum degree is 100mbar), keep the constant temperature reactor state until the amount of solvent collected in the solvent condenser reaches the added amount 1 / 3, close the 8#, 9# valves and the vacuum distillation device; open the 7#, 4...
Embodiment 2
[0075] (1) Open the 2# and 11# valves, add DMC to the constant temperature reactor, the amount added is 1 / 3 of the reactor volume; open the 7# valve, turn on the condensation reflux device, and add BF from the liquid feeding port 2# 3 ·DMC (DMC and BF 3 ·The molar ratio of DMC is 1:2), after stirring, close the 11# valve.
[0076] (2) Open the 1# valve of the solid feeding port, and add Li to the mixed solution 2 C 2 o 4 After closing the valve, its BF 3 ·The molar ratio of DMC was 1:3; the temperature was raised to 60°C, and the reaction was heated under reflux for 12 hours.
[0077] (3) Close the 7# valve and the condensing reflux device, open the 8# and 9# valves, start the vacuum distillation device (vacuum degree is 300mbar), keep the constant temperature reactor state until the amount of solvent collected in the solvent condenser reaches the amount added 1 / 2, close 8#, 9# valves and vacuum distillation devices; open 7#, 4# and 6# valves, open the condensation reflux d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com