Method and system for calculating tumor neoantigen load
An antigen and tumor technology, applied in biochemical equipment and methods, biostatistics, microbial measurement/testing, etc., can solve problems such as inconsistent thresholds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052] The preferred embodiments of the present invention will be described below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
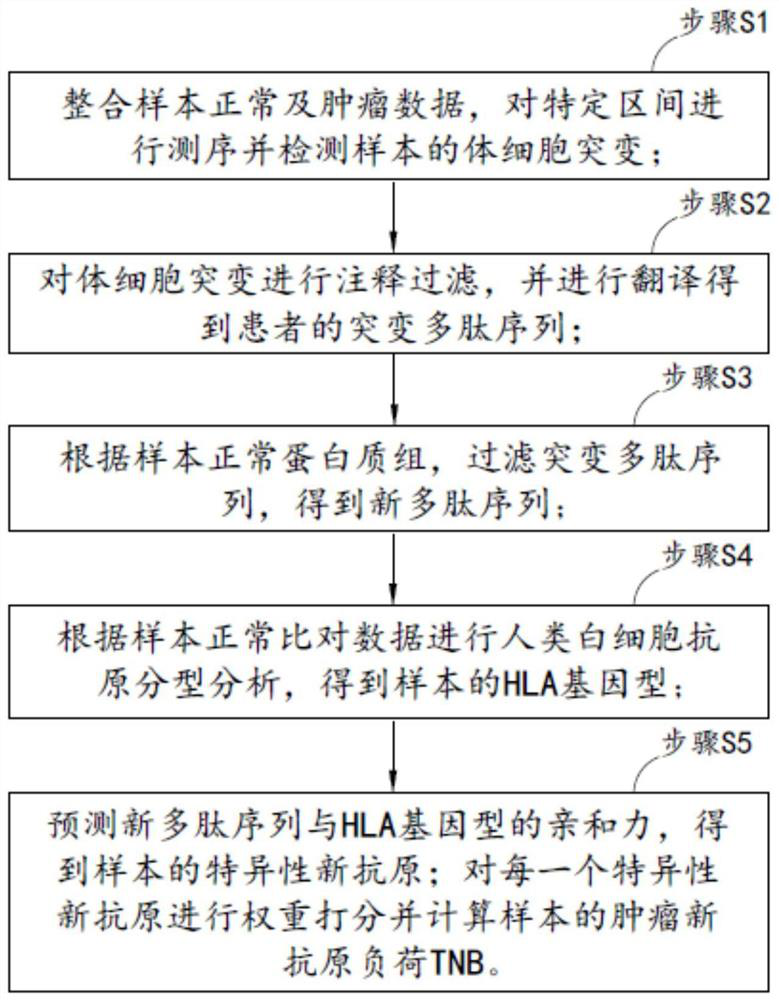
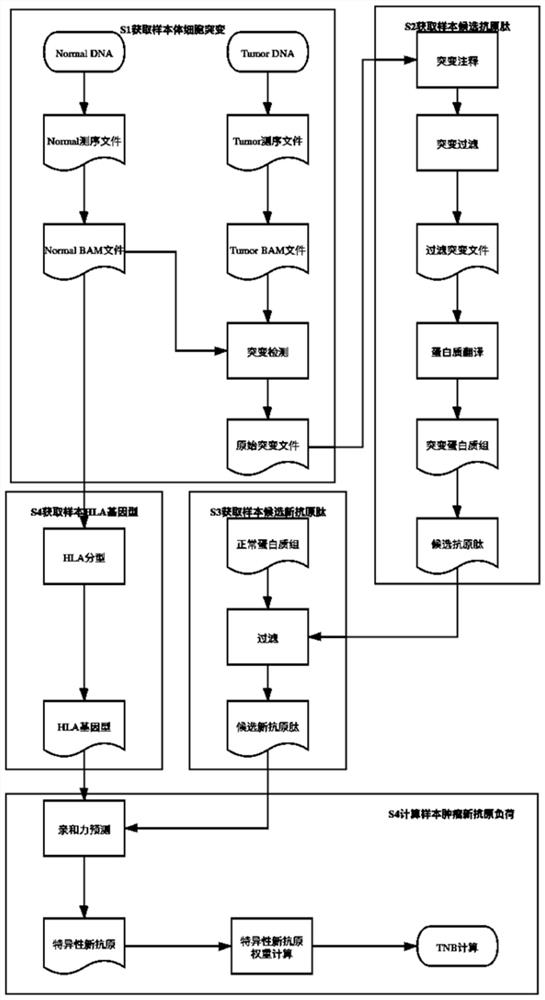
[0053] The embodiment of the present invention provides a method for calculating tumor neoantigen load, such as figure 1 shown, including:
[0054] Step S1: Integrate the normal and tumor data of the sample, sequence the specific interval and detect the somatic mutation of the sample;
[0055] Step S2: Annotate and filter somatic mutations, and translate to obtain the patient's mutant polypeptide sequence;
[0056]Step S3: According to the normal protein group of the sample, filter the mutant polypeptide sequence to obtain a new polypeptide sequence;
[0057] Step S4: Perform human leukocyte antigen typing analysis according to the sample normal comparison data to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com