Acylthiourea neuraminidase inhibitor as well as preparation and application thereof
A technology of neuraminidase and acylthiourea, which is applied in the directions of antiviral agents and organic chemistry, can solve the problems of expensive raw materials for Tamiflu production and complex synthesis process, and achieves good neuraminidase inhibitory activity and simple synthesis method. , the effect of excellent neuraminidase inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
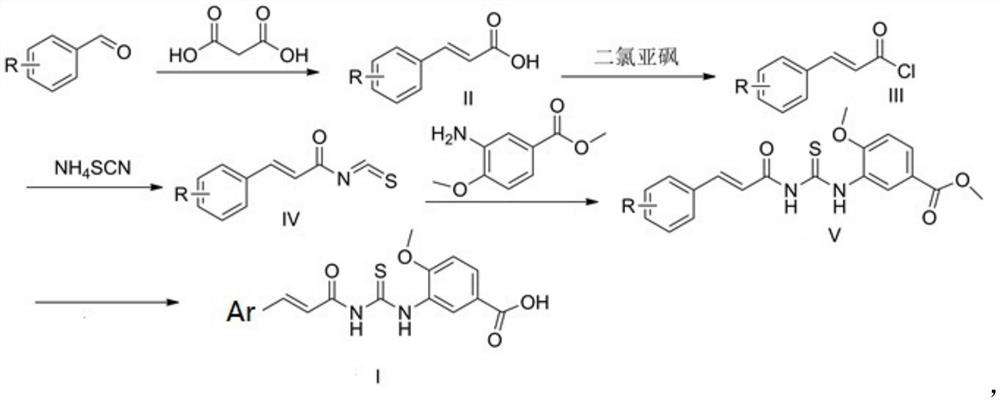
[0046] Described preparation method specifically comprises the following steps:
[0047] (1) substituted benzaldehyde and malonic acid are formed into a reaction system, and after the reaction, the intermediate of formula (II) is obtained through aftertreatment;
[0048] (2) The intermediate of formula (II) obtained in step (1) and thionyl chloride are dissolved in an organic solvent to form a reaction system, and the intermediate of formula (III) is obtained after the reaction;
[0049] (3) the intermediate of formula (III) obtained in step (2) and ammonium thiocyanate are dissolved in an organic solvent to form a reaction system, and after the reaction, the intermediate of formula (IV) is obtained through aftertreatment;
[0050] (4) The formula (IV) intermediate obtained in step (3) and 3-amino-4-methoxybenzoic acid methyl ester are dissolved in an organic solvent to form a reaction system, and after the reaction, the intermediate of formula (V) is obtained through post-tre...
Embodiment
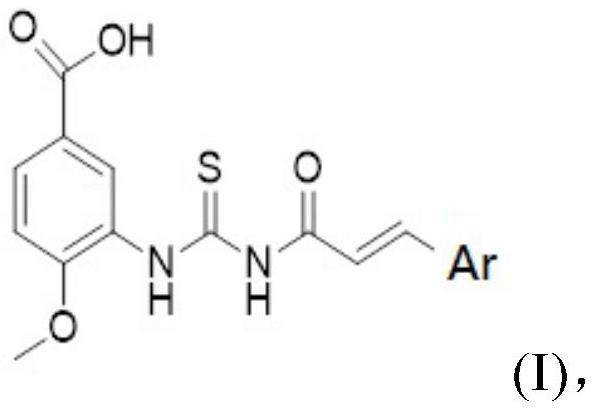
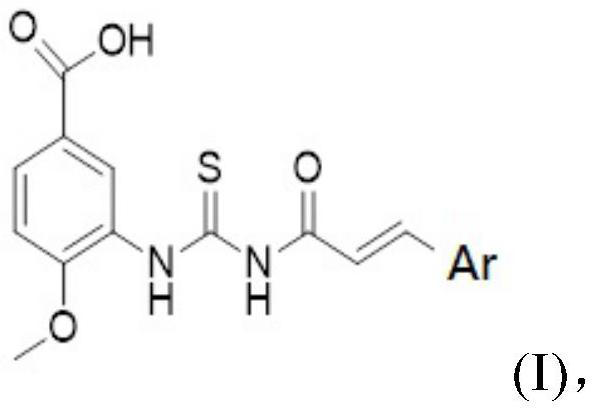
[0059] A preparation method containing an acylthiourea neuraminidase inhibitor, the structural formula of which is shown in Formula A:
[0060] That is, Ar is
[0061] Concrete synthetic steps are as follows:
[0062] (1) Accurately weigh 1.67g (10mmol) of 3-hydroxyl-4-nitrobenzaldehyde and 1.04g (10mmol) of malonic acid in a 50mL round bottom flask, add 20mL absolute ethanol, then add dropwise a catalytic amount (1 drops) of piperidine. After the dropwise addition, take out the round-bottomed flask, place it in a constant temperature oil bath at 85°C to heat and stir for 2 hours, take it out and cool the reaction solution, adjust the pH to 3 with hydrochloric acid and let stand to precipitate a large amount of precipitate, filter the precipitate to obtain a filter cake, filter The cake is washed many times with a large amount of ice water, then dried in a vacuum oven, and recrystallized from ethanol to obtain the intermediate of formula (II) (wherein Ar is ).
[0063...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com