In-vitro lung mechanical perfusate, preparation method and application thereof
A perfusate and lung technology, applied in the field of isolated lung mechanical perfusate and its preparation, can solve the problems of high price, difficulty in effective treatment, single product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Dissolve human serum albumin, polysucrose 70, dextran 40, mannitol, and nitroglycerin in DMEM / F12 (1:1) medium according to the concentrations in the table below, and then adjust the pH to 7.4 with sodium hydroxide.
[0035] components Concentration(g / L) human serum albumin 10 Ficoll 70 60 Dextran 40 10 Mannitol 7.2 Nitroglycerin 10
[0036] The isolated lung mechanical perfusion fluid prepared in Example 1 was used for animal experiments.
[0037] Anesthesia and weighing: Intramuscularly inject 2ml of atropine to the pigs used in the experiment, and then inject 2ml of Sotapine 15 minutes later. After the pigs are stabilized, they are weighed, and the weight is 30.0kg. An intravenous line was established and propofol was administered continuously intravenously.
[0038] Chest opening: endotracheal intubation, after breathing support, the chest cavity is opened.
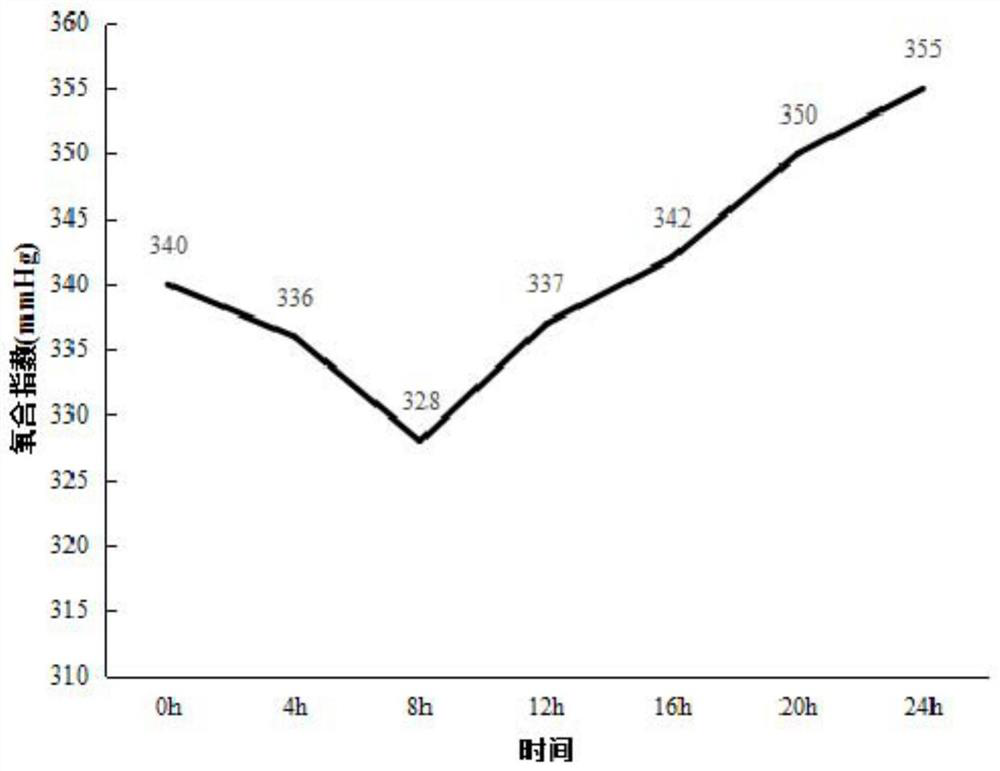
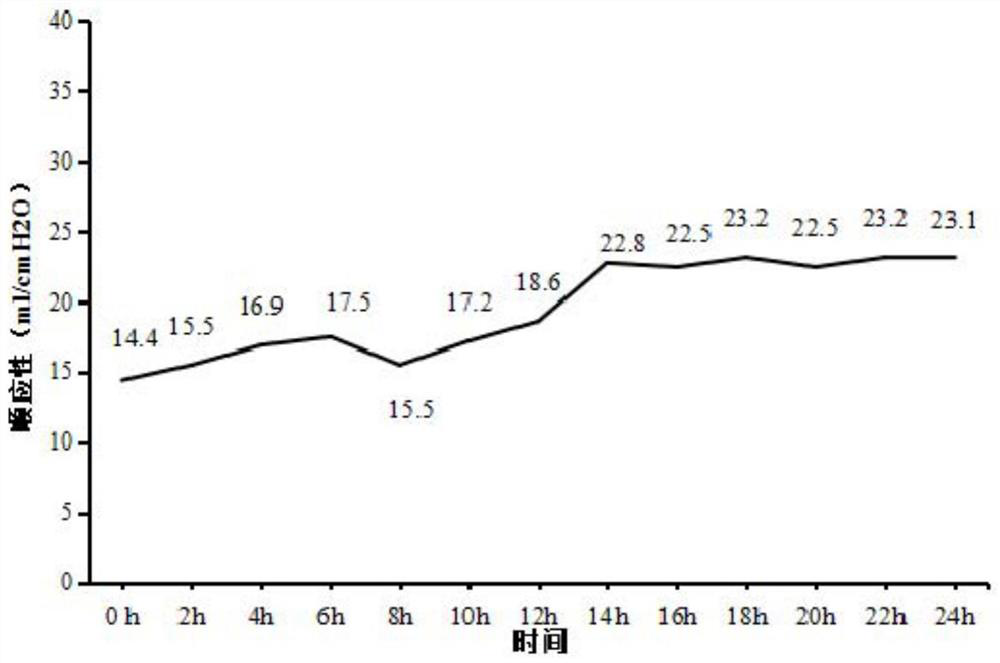
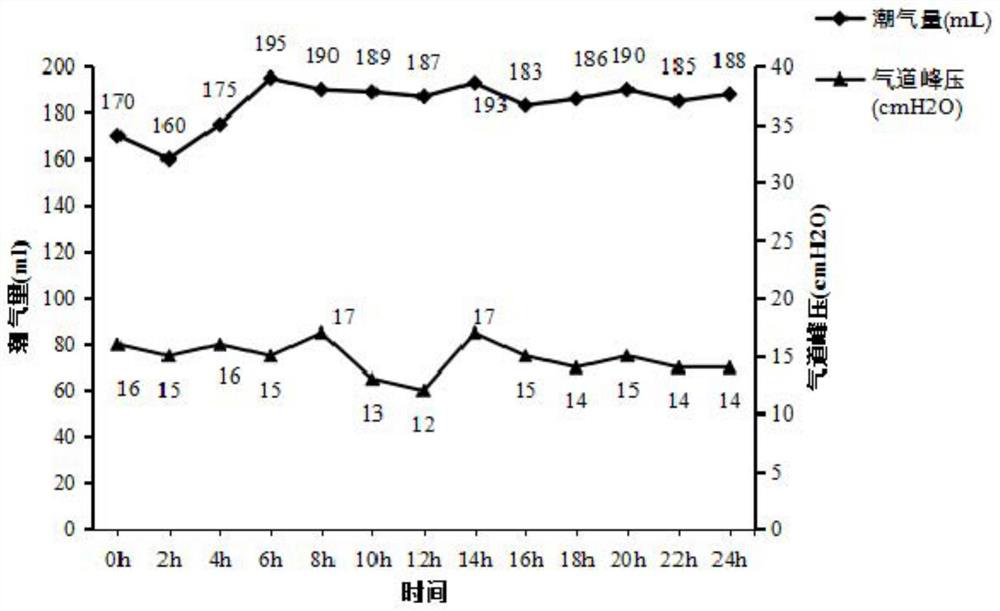
[0039] In vivo perfusion of the lung: 10×12 special-shaped jo...
Embodiment 2
[0047] Dissolve human serum albumin, polysucrose 70, dextran 40, mannitol, and nitroglycerin in DMEM / F12 (1:1) medium according to the concentrations in the table below, and then adjust the pH to 7.4 with sodium hydroxide.
[0048]
[0049]
[0050] The isolated lung mechanical perfusion solution prepared in Example 2 was used to repair the isolated lung perfusion, which can be maintained for 8 hours without edema in the lung.
Embodiment 3
[0052] Dissolve human serum albumin, polysucrose 70, dextran 40, mannitol, and nitroglycerin in DMEM / F12 (1:1) medium according to the concentrations in the table below, and then adjust the pH to 7.4 with sodium hydroxide.
[0053] components Concentration(g / L) human serum albumin 8 Ficoll 70 65 Dextran 40 15 Mannitol 10 Nitroglycerin 15
[0054] The isolated lung mechanical perfusion solution prepared in Example 3 was used to repair the isolated lung perfusion, which can be maintained for 18 hours without edema in the lung.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com