Anti-PD-L1 nano antibody as well as derivative and application thereof
A PD-L1 and nanobody technology, applied in the field of biomedicine or biopharmaceuticals, can solve the problems of small molecular weight, lack of nanobody, stable immunogenicity and weak penetrability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1: Construction of Nanobody Library
[0193] animal immunity
[0194] Mix 1 mg of human PD-L1 antigen (purchased from AcroBiosystems) with an equal volume of Freund's adjuvant, immunize 2 alpacas (Llama), once a week, and immunize 4 times in total to stimulate B cells to express antigen-specific nanobodies . After the 4 times of immunization, 50ml of alpaca peripheral blood was extracted, and lymphocytes were separated by lymphocyte separation medium. Total RNA was extracted using RNA extraction reagent Trizol (purchased from Invitrogen). Alpaca total cDNA was obtained by reverse transcription using a cDNA synthesis kit (purchased from Invitrogen).
[0195] Nanobody gene amplification
[0196] In the first round of PCR, the IgG2 and IgG3 sequences were amplified from the cDNA:
[0197] Table 1. First-round PCR primers
[0198] name sequence (5' to 3') upstream primer GTCCTGGCTGCTCTTCTACAAGG downstream primer GGTACGTGCTGTTGAACT...
Embodiment 2
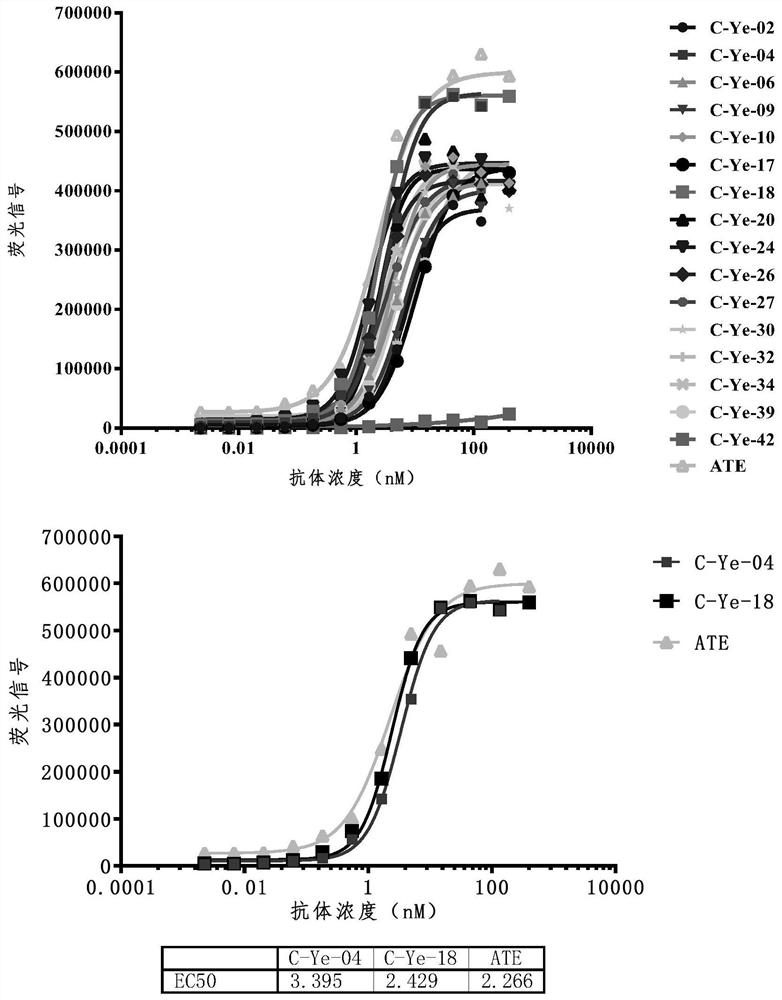
[0208] Example 2: Screening of PD-L1 Nanobodies
[0209] Biotinylation of human PD-L1 protein
[0210] Take an appropriate volume of double distilled water to dissolve human PD-L1 protein (purchased from AcroBiosystems), follow the product instructions of the biotin labeling kit (purchased from Thermo), dissolve the biotin and mix it with the protein solution, and incubate at 4°C for 2 hours. A desalting column (purchased from Thermo) was used to remove excess biotin, and the pretreatment of the desalting column and sample collection operations were performed referring to the steps in the product manual.
[0211] MACS enrichment of yeast that specifically binds to PD-L1
[0212] The VHH library constructed in Example 2 was inoculated in SD-CAA expansion medium (6.7g YNB, 5g casamino acids, 13.62g Na 2 HPO 4 12H 2 O, 7.44g NaH 2 PO 4 and 2% glucose), the number of yeast cells inoculated >10× library capacity (initial amplification concentration = 0.5OD 600 / ml), 30°C...
Embodiment 3
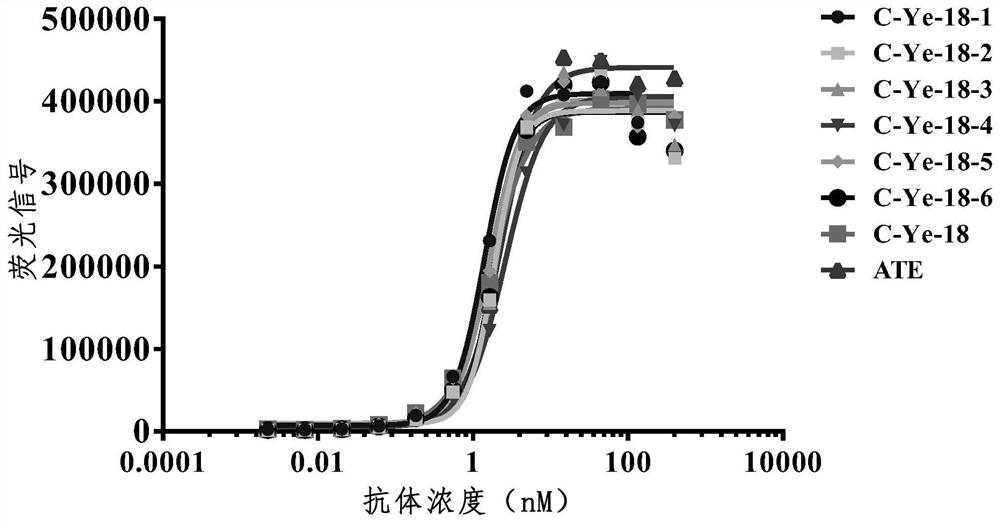
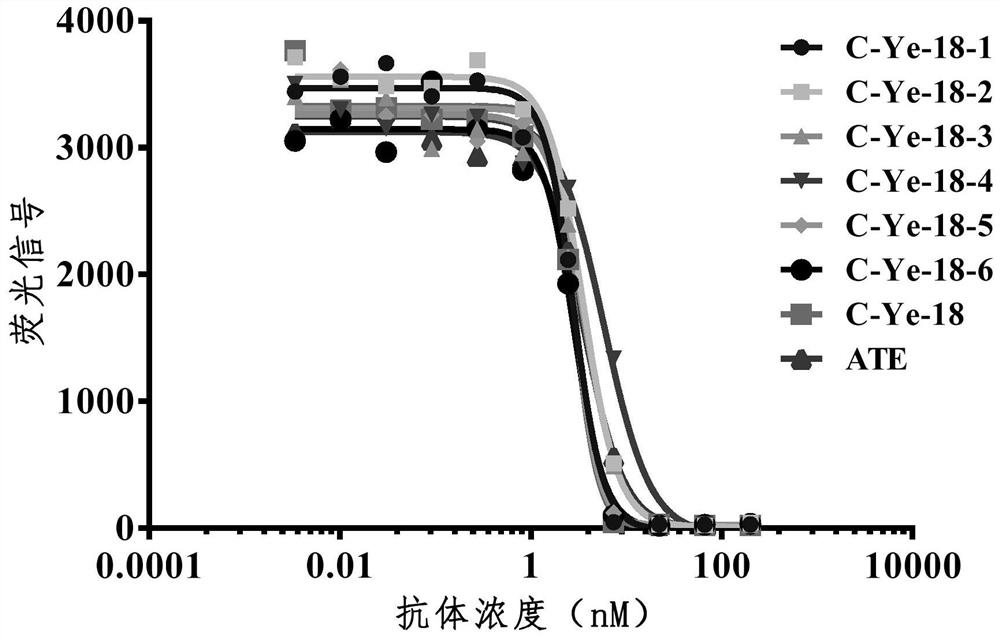
[0218] Example 3: Construction, expression and purification of heavy chain antibodies
[0219] Antibody gene construction into pCDNA3.1 expression vector
[0220] The VHH gene sequence was connected to the Fc segment of human IgG1 (LALA mutation), and the linearized pCDNA3.1 vector was digested with homologous recombinase (purchased from Vazyme) and EcoR I / Not I double restriction enzymes, and the procedure was in accordance with the product instructions. Homologous recombination products were transformed into Top10 competent cells, coated with ampicillin-resistant plates, cultured at 37°C overnight, and single clones were picked and sequenced.
[0221] cell transfection
[0222] Using ExpiCHO TMExpression system kit (purchased from Thermo), the plasmid was transferred into Expi-CHO cells, the transfection method was according to the product instructions, and the supernatant was collected after 5 days of cell culture and purified by protein A magnetic beads (purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com