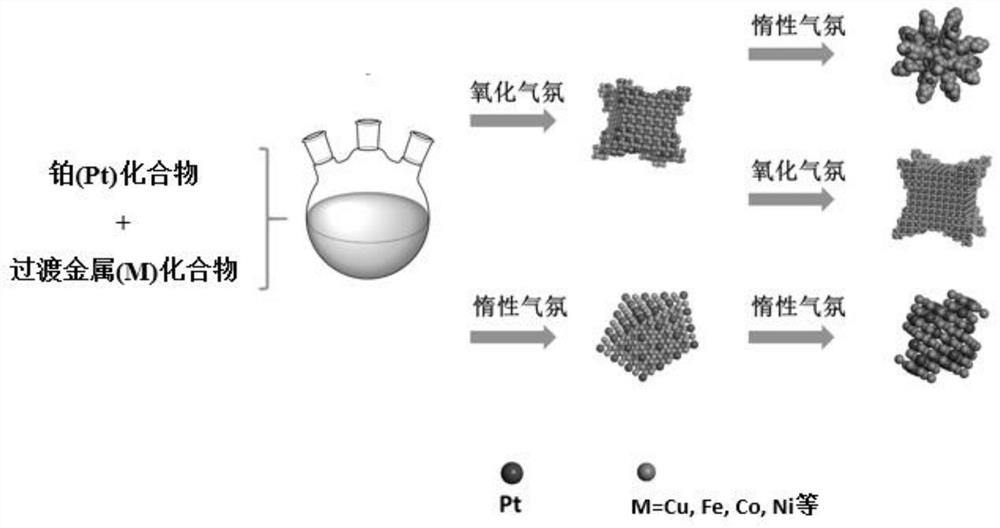
Core-shell platinum-based alloy electrocatalyst with high oxygen reduction performance and preparation method thereof
A technology of electrocatalysts and platinum-based alloys, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of block copolymers being difficult to wash and remove, and the reduction of electrocatalytic activity, and achieve novel and unique preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
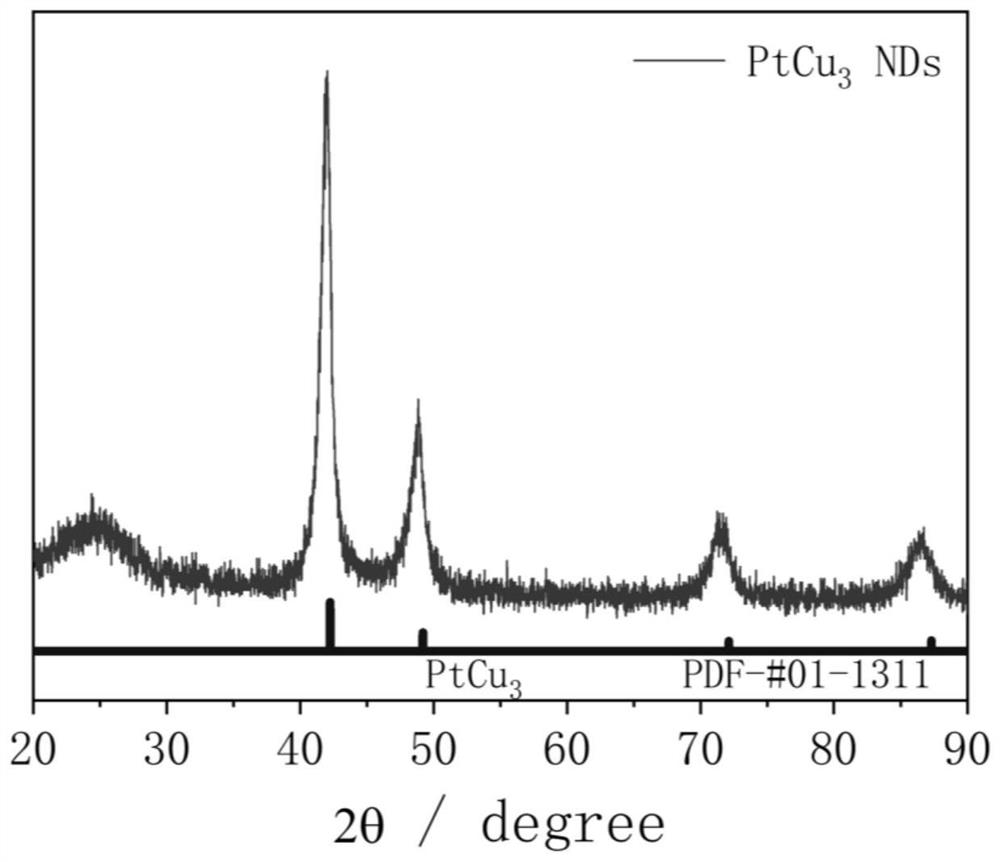
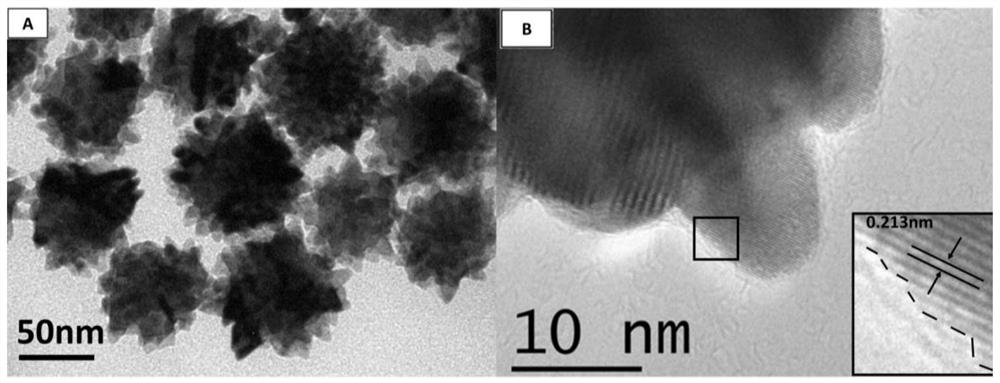
[0038] 1) PtCu 3 Preparation of nanodendrites
[0039]Inject 400 μL of a mixed solution of chloroplatinic acid and copper nitrate (containing 0.038 mmol of chloroplatinic acid and 0.114 mmol of copper nitrate) into a three-necked flask containing 10 mL of oleylamine, and heat and stir at 120 °C for 10 min to remove water in the system. Afterwards, air was introduced into the bottle, and the three-necked flask was transferred to a 270°C oil bath. After 5 minutes, the air was replaced with argon, the reaction was continued for 25 minutes, the reaction was stopped, and cooled to room temperature. Centrifuged and washed 5 times with n-hexane to obtain PtCu 3 nano dendrites. Its general preparation process is as follows figure 1 shown.
[0040] 2) Structural composition analysis of the catalyst
[0041] Combining multiple characterization techniques for the preparation of PtCu 3 The structural composition of nanodendritic catalysts was analyzed in depth: ICP-AES gave the ato...
Embodiment 2
[0051] 1) Preparation of PtCu nanodendrites
[0052] Inject 400 μL of a mixed solution of chloroplatinic acid and copper nitrate (containing 0.076 mmol of chloroplatinic acid and 0.076 mmol of copper nitrate) into a three-necked flask containing 10 mL of oleylamine, and heat and stir at 120 °C for 10 min to remove water in the system. Afterwards, air was introduced into the bottle, and the three-necked flask was transferred to a 270°C oil bath. After 5 minutes, the air was replaced with argon, the reaction was continued for 25 minutes, the reaction was stopped, and cooled to room temperature. The PtCu nanodendrites were obtained by centrifuging and washing 5 times with n-hexane. Its TEM characterization results are as follows Figure 7 As shown, the size of the formed nanodendrites is more uniformly distributed.
[0053] 2) Carbon loading and electrochemical dealloying of PtCu nanodendrites
[0054] The prepared PtCu nano dendrites were dispersed in isopropanol, Vulcan XC-...
Embodiment 3
[0060] 1) Pt 3 Preparation of Cu nanodendrites
[0061] Inject 400 μL of a mixed solution of chloroplatinic acid and copper nitrate (containing 0.114 mmol of chloroplatinic acid and 0.038 mmol of copper nitrate) into a three-necked flask containing 10 mL of oleylamine, and heat and stir at 120 °C for 10 min to remove water in the system. Afterwards, air was introduced into the bottle, and the three-necked flask was transferred to a 270°C oil bath. After 5 min the air was replaced with argon. React for another 25 min, stop the reaction, and cool to room temperature. Centrifuged and washed 5 times with n-hexane to obtain Pt 3 Cu nanodendrites. Its TEM characterization results are as follows Figure 8 As shown, the size of the formed nanodendrites is more uniformly distributed.
[0062] 2) Pt 3 Carbon loading and electrochemical dealloying of Cu nanodendrites
[0063] The resulting Pt 3 Cu nano dendrites were dispersed in isopropanol, Vulcan XC-72R conductive carbon blac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com