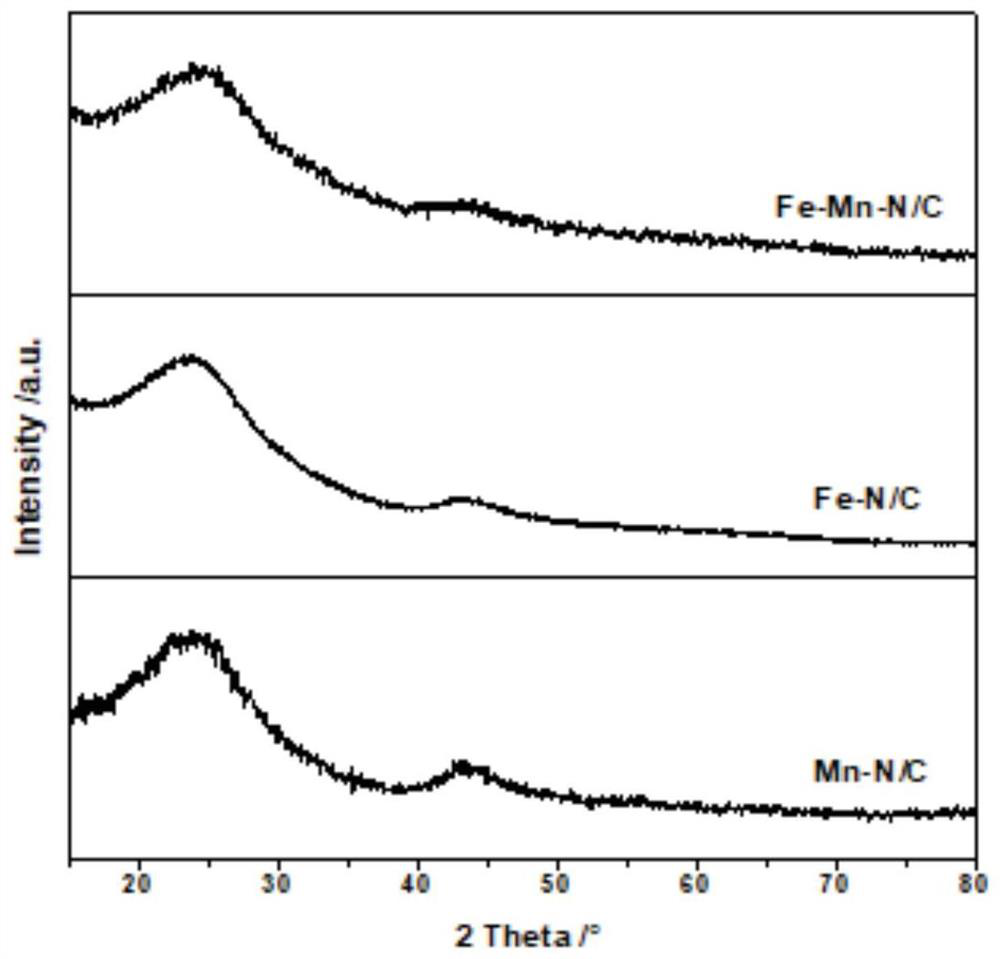
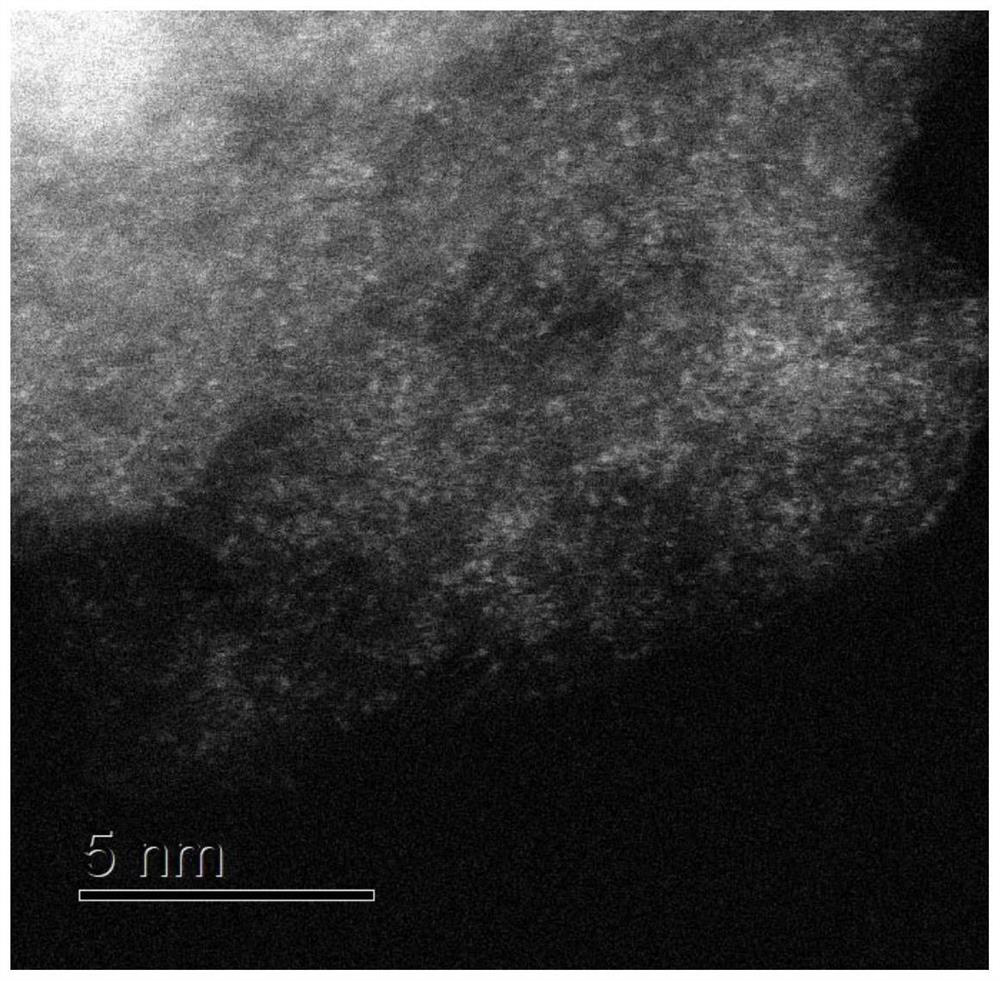
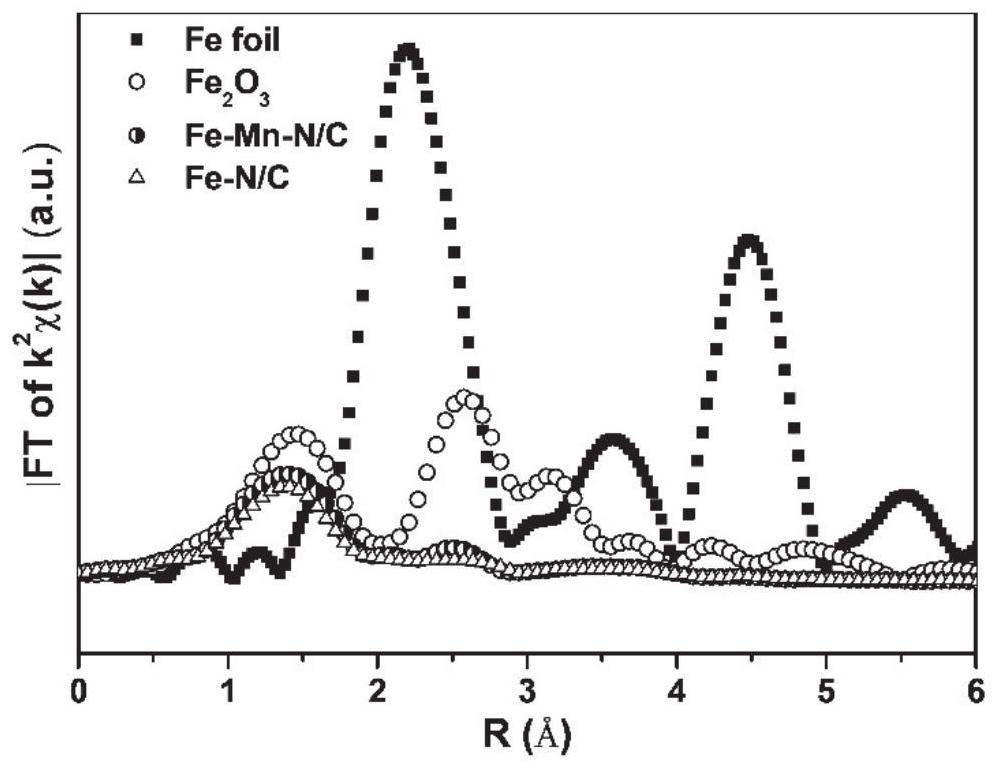
Carbon-based bimetallic Fe-Mn monatomic electrocatalyst, preparation method and application thereof
An electrocatalyst, bimetallic technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of low activity and poor stability, and achieve high efficiency oxygen reduction, high dispersion, and strong controllability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Preparation of catalyst:
[0042] a) Weigh 3g of carbon black (black pearls 2000, denoted as BP 2000) into a 200mL beaker, add 150mL of concentrated nitric acid (65-68%), stir at room temperature for 10h, and let stand overnight. The filtered solid was washed several times with deionized water until the washing solution was neutral, and then dried in an oven at 60°C overnight to obtain surface-functionalized BP 2000.
[0043] b) Take 0.15 g of surface-functionalized BP 2000, add 50 mL of absolute ethanol, and sonicate for 30 min. Add 0.047g of iron acetate and 0.067g of manganese acetate (the molar ratio of Fe and Mn is 1:1), fully stir to dissolve, add 0.32g of 1,10-phenanthroline, and stir for 20min. Weigh 0.5g of MgO and 30mL of absolute ethanol into the above mixed solution. Then the above suspension was vigorously stirred in a 60°C water bath for 10 hours, then evaporated to dryness in a water bath at 60°C, and dried overnight in a 60°C water bath to obtain Fe...
Embodiment 2
[0055] Catalyst preparation:
[0056] a) Weigh 3g of carbon black (black pearls 2000, denoted as BP 2000) into a 200mL beaker, add 150mL of concentrated nitric acid (65-68%), stir at room temperature for 10h, and let stand overnight. The filtered solid was washed several times with deionized water until the washing solution was neutral, and then dried in an oven at 60°C overnight to obtain surface-functionalized BP 2000.
[0057] b) Take 0.15 g of surface-functionalized BP 2000, add 50 mL of absolute ethanol, and sonicate for 30 min. Add 0.075g of iron acetate and 0.027g of manganese acetate (the molar ratio of Fe and Mn is 4:1), fully stir to dissolve, add 0.32g of 1,10-phenanthroline, and stir for 20min. Weigh 0.5g of MgO and 30mL of absolute ethanol into the above mixed solution. Then the above suspension was vigorously stirred in a 60°C water bath for 10 hours, then evaporated to dryness in a water bath at 60°C, and dried overnight in a 60°C water bath to obtain Fe, Mn(p...
Embodiment 3
[0062] Catalyst preparation:
[0063] a) Weigh 3g of carbon black (black pearls 2000, denoted as BP 2000) into a 200mL beaker, add 150mL of concentrated nitric acid (65-68%), stir at room temperature for 10h, and let stand overnight. The filtered solid was washed several times with deionized water until the washing solution was neutral, and then dried in an oven at 60°C overnight to obtain surface-functionalized BP 2000.
[0064] b) Take 0.15 g of surface-functionalized BP 2000, add 50 mL of absolute ethanol, and sonicate for 30 min. Add 0.019g of iron acetate and 0.107g of manganese acetate (the molar ratio of Fe and Mn is 1:4), fully stir to dissolve, add 0.32g of 1,10-phenanthroline, and stir for 20min. Weigh 0.5g of MgO and 30mL of absolute ethanol into the above mixed solution. Then the above suspension was vigorously stirred in a 60°C water bath for 10 hours, then evaporated to dryness in a water bath at 60°C, and dried overnight in a 60°C water bath to obtain Fe, Mn(p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com